Introduction
Ask any person to name the things in life that bring them pleasure, and chances are good that they will mention music. Music is a universal source of enjoyment for humans, and hearing a favorite tune can instantly lift one’s mood. It may even induce physical effects, such as tears, goosebumps, or tingling sensations down the spine called “chills.” Music has such a strong effect on human emotion that people invest significant amounts of time and money into buying music and going to musical events. The positive responses induced by music are comparable to those generated by food and sex, but unlike those stimuli, music has no known biological significance. It is puzzling, then, why people find listening to music so rewarding. In order to better understand why these responses occur, it is important to first understand how music induces such pleasure.
Musicians’ Responses to Music
An early study to investigate the specific neural correlates of chills in response to music was conducted by Blood and Zatorre, using musicians as subjects [1] . The subjects, who each had at least eight years of experience playing an instrument beginning at various ages, were each asked to choose one instrumental classical piece that consistently induced chills. A ninety-second clip from each subject’s selected piece was played, and a clip from a different subject’s piece was used as a non-chill-inducing control. When subjects reported feeling tingling sensations while listening to their selected piece, their heart rate, muscular electrical activity, and respiration depth all increased in comparison to the control, supporting the idea that highly pleasurable music can induce physiological effects.
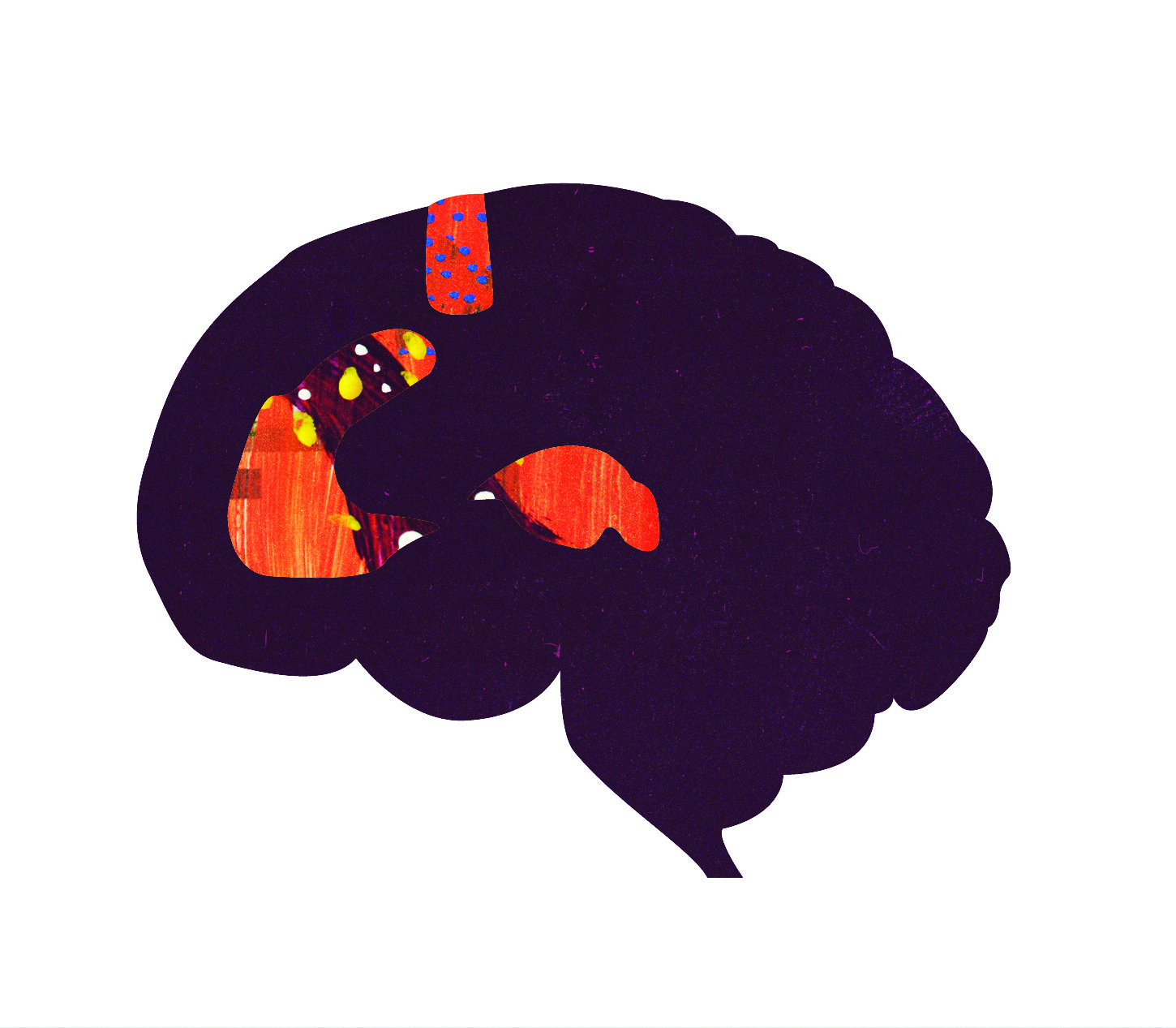
Using positron emission topography (PET), the researchers measured blood flow to brain regions of participants while they listened to the chill-inducing clips. An increase in blood flow suggests increased activity in those specific regions. During positive responses, flow increased to the left ventral striatum, left dorsomedial midbrain, right thalamus, anterior cingulate, supplementary motor area, and left cerebellum. These areas are known to be involved in reward processing [2] and arousal [3] , and these activation patterns are similar to those produced by food, sex, and certain drugs [4-6] . Blood flow was decreased to the right amygdala and and ventral medial prefrontal cortex, regions associated with feelings of aversion [2] . This suggests that pleasurable responses to music are modulated not only by increasing activation in brain regions associated with positive emotions, but also by decreasing activation in those associated with negative emotions. Interestingly, the amygdala has been implicated in generating feelings of fear and aversion [7, 8] , but has also been shown to be activated during pleasure [5] . Its role appears to be more complicated, requiring further investigation.
The regions found by Blood and Zatorre to be activated during positive responses to music differ from those activated during perception of its components, such as pitch and rhythm [9-11] . Such results suggest that there may be some separation between the processes involved in the perception of music and the emotional responses to music. Alternatively, emotional responses may rely on additional processes that build upon those involved in musical perception. Such ideas are further supported by a case study in which a patient with cortical neurodegeneration could not perceive many components of music—he could not recognize highly familiar melodies he was played, such as the United States National Anthem, nor was he able to differentiate timbres of instruments used in the selections [12] . Nonetheless, the patient still reported deriving pleasure from listening to the music [12] .
Non-Musicians’ Responses to Music
While Blood and Zatorre’s study identified the specific neural systems involved in pleasurable responses to music, one of its limitations was that its participants were all musicians. There is reason to think that, neurologically, non-musicians can experience musical pleasure differently than musicians, since other dissimilarities between the two groups have been demonstrated. For instance, distinct brain regions are activated in musicians versus on-musicians when listening to music. Both musicians and non-musicians demonstrate activation in the temporal lobe (which includes auditory processing areas) during passive music listening, but musicians show dominant activation in the left temporal lobe, while non-musicians show right-dominant temporal lobe activation [13] . Musicians also display higher levels of activation in other auditory association areas, such as the planum temporale [13] .
Studies of positive responses to music in non-musicians showed some results similar to those obtained with musicians. In a study conducted by Brown et al., subjects with minimal musical experience were played unfamiliar but pleasant Greek instrumental music [14] . The general brain regions activated were similar to those activated during highly pleasurable responses in musicians, but the exact activated areas differed [14] . While these differences may have been due to the genre of the music, the researchers hypothesized that they more likely resulted from the apparent lack of a chills response, as self-reported by the participants. This was possibly due to the unfamiliarity of the music used in this study.
The differences between responses in musicians and non-musicians can be better seen in a functional magnetic resonance imaging (fMRI) study conducted by Chapin et al. The fMRI measured the neural responses of both groups to the same classical piano piece [15] . Although the areas activated during self-reported pleasurable responses were the same for all subjects, musicians showed a greater level of activation in the ventral anterior cingulate, a region involved in emotional processing [15, 16] . The researchers suggested that this increased activation resulted from more robust connections between brain regions involved in musical perception and emotional responses in musicians. It is not clear if these strengthened interactions were caused by their experience in playing music, or if it is the other way around—as the researchers put it, “Musical involvement may lead to enhanced neural responses, or alternatively, enhanced emotional responding to music may lead one to seek out musical activities” [15] . More research is needed to establish a more certain cause-and-effect relationship.
Although these studies have shown that there are some differences in how extensively musical pleasure is processed in the brains of musicians and non-musicians, the general neural circuits activated appear to be the same across both groups—namely, those involved with emotion and reward [15] . These areas facilitate such responses through neurotransmitter release.
Neurochemical Mechanisms
The neurotransmitter dopamine, known as a “happy hormone,” is an important mediator of enjoyable responses [17] . It is not surprising, then, that it plays a key role in inducing musical pleasure. In a PET experiment conducted by Salimpoor et al., subjects with a wide range of musical training were played self-selected, highly-pleasurable instrumental pieces [18] . Increased dopamine release in the ventral striatum and nucleus accumbens was detected during chills responses, indicating that dopamine contributes to generating these feelings [18] . The researchers did not distinguish between results obtained from experienced versus inexperienced subjects, so further experiments are needed to determine if there are differences in dopamine release levels in the two groups, as there are in activation levels.

Opioids , a class of neurotransmitters that includes endorphins, have also been shown to mediate pleasurable responses to stimuli such as food [19] . Many drugs, such as morphine and oxycodone, target the opioid receptor system, stimulating intense feelings of pleasure that can lead to addiction [20] . While the role of opioids in mediating responses to music has not been as extensively studied as that of dopamine, there is some evidence to suggest that they, too, play a part. Music has been shown to lower the need for opioid receptor-binding painkillers in post-operative patients, suggesting that listening to music may increase endogenous release of opioids [21] . Opioid blockers have also been shown to block the chills response to music [22] . However, the study in which this was observed used very few subjects and lacked a non-music control, limiting interpretation of its results [22] . Direct imaging of opioid receptors is needed to define more clearly their role in creating positive responses to music [23] .
The Role of Familiarity
Often, a piece of music that does not strongly appeal to a listener upon first listen can later “grow on” them after repeated exposure. Dopamine plays a role in this phenomenon, which was demonstrated in a study by Van den Bosch et al. in which increased familiarity with a specific piece of music (or “explicit familiarity”) was correlated with increases in the listener’s pleasure [24] . In other words, listeners who felt neutral towards a piece at first found it more enjoyable after hearing it multiple times. It is not known if this effect also occurs when listeners negatively respond to a piece upon first listen, so the role of initial impressions remains to be seen. Nonetheless, the demonstrated effects of familiarity make sense—music is made up of patterns, and in response to a familiar pattern, dopamine can be released to increase pleasurable feelings [25, 26] . Such release was detected by Salimpoor et al. in subjects listening to chill-inducing music. Prior to the occurrence of the chills, dopamine release was detected in the caudate, while release in the nucleus accumbens was detected during the chills themselves [18] . This separate, anticipatory release of dopamine in the caudate was thought to heighten the listeners’ pleasure even more, according to the researchers. However, only responses to familiar music were recorded in this study. In a later study also conducted by Salimpoor et al., subjects showed some anticipatory dopamine release in similar regions even in response to unfamiliar, but pleasant, music [27] . Such unexpected responses could be attributed to a different type of familiarity: implicit familiarity.
Implicit familiarity is the familiarity with the structure of a certain genre of music—for example, the types of scales it uses, or its typical rhythmic pattern [25] . This familiarity can be derived from past exposure to the genre, as well as being trained to play it [25, 28, 29] . A listener’s culture can also play a role in shaping their implicit familiarity, as musical structures vary widely across cultures [30] . Implicit familiarity could help explain the results of the previously-mentioned experiment—the unfamiliar pieces that induced anticipatory dopamine release were those deemed enjoyable by the subjects according to their personal preference, implying that they were familiar with the style of the music [28] . The music in the experiment included vocals, however, which can activate additional regions of the brain [31] . More research studying the responses to music alone is needed to properly account for the role of implicit familiarity, as well as how it can interact with explicit familiarity to heighten musical pleasure.
Conclusion
The use of brain imaging technologies has helped to paint a more complete picture of how music affects the human brain and generates such strong emotions. However, many questions remain about the details of the process and how it can be affected by an individual’s personal experiences with music. The evolutionary motivation behind the development of these responses is also still mostly shrouded in mystery. Nonetheless, current research has shown that music can play an important biological role by increasing positive emotions and decreasing negative ones in humans, improving their emotional state and possibly even their health. The observed effects have even been demonstrated in animals [32] . This evidence suggests that music could function as a therapeutic agent, and future research may help to illuminate how such a simple pleasure could serve a more vital role.
- Blood AJ, Zatorre RJ. (2001). Intensely pleasurable responses to music correlate with activity in brain regions implicated in reward and emotion. Proc Natl Acad Sci USA. 98(20):11818-23.
- Bardo MT. (1998). Neuropharmacological mechanisms of drug reward: beyond dopamine in the nucleus accumbens. Crit Rev Neurobiol. 12(1-2):37-67.
- Paus T. (2000). Functional anatomy of arousal and attention systems in the human brain. Prog Brain Res. 126:65-77.
- Pfaus JG, Damsma G, Wenkstern D, Fibiger HC. (1995). Sexual activity increases dopamine transmission in the nucleus accumbens and striatum of female rats. Brain Res. 693(1-2):21-30.
- Schilström B, Svensson HM, Svensson TH, Nomikos GG. (1998) Nicotine and food induced dopamine release in the nucleus accumbens of the rat: putative role of alpha7 nicotinic receptors in the ventral tegmental area. Neuroscience. 85(4):1005-9.
- Berridge KC. (2003). Pleasures of the brain. Brain Cogn. 52(1):106-28.
- Zald DH, Pardo JV. (1997). Emotion, olfaction, and the human amygdala: amygdala activation during aversive olfactory stimulation. Proc Natl Acad Sci USA.;94(8):4119-24.
- Adolphs R, Tranel D, Damasio H, Damasio AR. (1995). Fear and the human amygdala. J Neurosci. 15(9):5879-91.
- Blood AJ, Zatorre RJ, Bermudez P, Evans AC. (1999). Emotional responses to pleasant and unpleasant music correlate with activity in paralimbic brain regions. Nat Neurosci. 2(4):382-7.
- Zatorre RJ, Samson S. (1991). Role of the right temporal neocortex in retention of pitch in auditory short-term memory. Brain. 114 ( Pt 6):2403-17.
- Chen JL, Penhune VB, Zatorre RJ. (2008). Listening to musical rhythms recruits motor regions of the brain. Cereb Cortex. 18(12):2844-54.
- Matthews BR, Chang CC, De May M, Engstrom J, Miller BL. (2009). Pleasurable emotional response to music: a case of neurodegenerative generalized auditory agnosia. Neurocase. 15(3):248-59.
- Ohnishi T, Matsuda H, Asada T, et al. (2001). Functional anatomy of musical perception in musicians. Cereb Cortex. 11(8):754-60.
- Brown S, Martinez MJ, Parsons LM. (2004). Passive music listening spontaneously engages limbic and paralimbic systems. Neuroreport. 15(13):2033-7.
- Chapin H, Jantzen K, Kelso JA, Steinberg F, Large E. (2010). Dynamic emotional and neural responses to music depend on performance expression and listener experience. PLoS ONE. 5(12):e13812.
- Phan KL, Wager T, Taylor SF, Liberzon I (2002) Functional neuroanatomy of emotion: A meta-analysis of emotion activation studies in PET and fMRI. Neuroimage 16: 331–348.
- Sharot, T., Shiner, T., Brown, A. C., Fan, J., & Dolan, R. J. (2009). Dopamine Enhances Expectation of Pleasure in Humans. Current Biology, 19(24):2077–2080.
- Salimpoor VN, Benovoy M, Larcher K, Dagher A, Zatorre RJ. (2011). Anatomically distinct dopamine release during anticipation and experience of peak emotion to music. Nat Neurosci. 14(2):257-62.
- Peciña S, Smith KS. (2010). Hedonic and motivational roles of opioids in food reward: implications for overeating disorders. Pharmacol Biochem Behav. 97(1):34-46.
- Kelley AE, Bakshi VP, Haber SN, Steininger TL, Will MJ, Zhang M. (2002). Opioid modulation of taste hedonics within the ventral striatum. Physiol Behav. 76(3):365-77.
- Cepeda MS, Carr DB, Lau J, Alvarez H. (2006). Music for pain relief. Cochrane Database Syst Rev. (2):CD004843.
- Goldstein, A. (1980). Thrills in response to music and other stimuli. Physiological Psychology, 8(1), 126-129.
- Chanda ML, Levitin DJ. (2013). The neurochemistry of music. Trends Cogn Sci (Regul Ed). 17(4):179-93.
- Van den bosch I, Salimpoor VN, Zatorre RJ. (2013). Familiarity mediates the relationship between emotional arousal and pleasure during music listening. Front Hum Neurosci. 7:534.
- Salimpoor VN, Zald DH, Zatorre RJ, Dagher A, Mcintosh AR. (2015). Predictions and the brain: how musical sounds become rewarding. Trends Cogn Sci (Regul Ed). 19(2):86-91.
- Howe MW, Tierney PL, Sandberg SG, Phillips PE, Graybiel AM. (2013). Prolonged dopamine signalling in striatum signals proximity and value of distant rewards. Nature. 500(7464):575-9.
- Salimpoor VN, Van den bosch I, Kovacevic N, Mcintosh AR, Dagher A, Zatorre RJ. (2013). Interactions between the nucleus accumbens and auditory cortices predict music reward value. Science. 340(6129):216-9.
- Pearce MT, Ruiz MH, Kapasi S, Wiggins GA, Bhattacharya J. (2010). Unsupervised statistical learning underpins computational, behavioural, and neural manifestations of musical expectation. Neuroimage. 50(1):302-13.
- Vuust P, Brattico E, Seppänen M, Näätänen R, Tervaniemi M. (2012). Practiced musical style shapes auditory skills. Ann N Y Acad Sci. 1252:139-46.
- Demorest SM, Osterhout L. (2012). ERP responses to cross-cultural melodic expectancy violations. Ann N Y Acad Sci. 1252:152-7.
- Escoffier N, Zhong J, Schirmer A, Qiu A. (2013). Emotional expressions in voice and music: same code, same effect?. Hum Brain Mapp. 34(8):1796-810.
- Bowman A, Scottish spca, Dowell FJ, Evans NP.(2015). ‘Four Seasons’ in an animal rescue centre; classical music reduces environmental stress in kennelled dogs. Physiol Behav. 143:70-82.
