Spoken Bird: The Curious Case of Parrot Mimicry
With about 11,000 species, birds make up one of the most diverse classes in the animal kingdom [1]. While one may be enchanted by the prehistoric-clawed hoatzin, the sashaying mating dance of the albatross, or the colorful flash from the bib of the Anna’s hummingbird, the parrot dazzles in various respects, making it one of the most extraordinary species to study. With the skill to visually stun anyone, coupled with its impressive intellectual potential, the parrot captivates humanity most through its ability to mimic human words. Why does the parrot speak? How can it speak when other songbirds—and animals—cannot? By delving into the structure, function, and genetics of the parrot brain, we can increase our understanding of this unique ability. While the causes of mimicry still remain a mystery, many theories pose promising hypotheses for this parrot trait, perhaps bringing us closer to understanding the complicated ins and outs of our own speech and its development.
Songbird Song Systems
The investigation into the curious nature of the parrot’s vocal ability starts with an analogous group of bird species with a similar talent: the songbird. While songbirds cannot mimic speech, they boast an impressive song learning system that is similar to the backbone of parrot speech structure [2]. This system consists of seven distinct nuclei with corresponding pathways that guide song production in the brain [3]. These nuclei are dubbed “song nuclei,” and they each have different specialized tasks [2]. The seven song nuclei are allocated into two major vocal pathways: the anterior forebrain pathway, which is in charge of vocal learning, and the posterior descending pathway, which is in charge of vocal production [4]. These pathways allow for communication between specific avian brain areas.
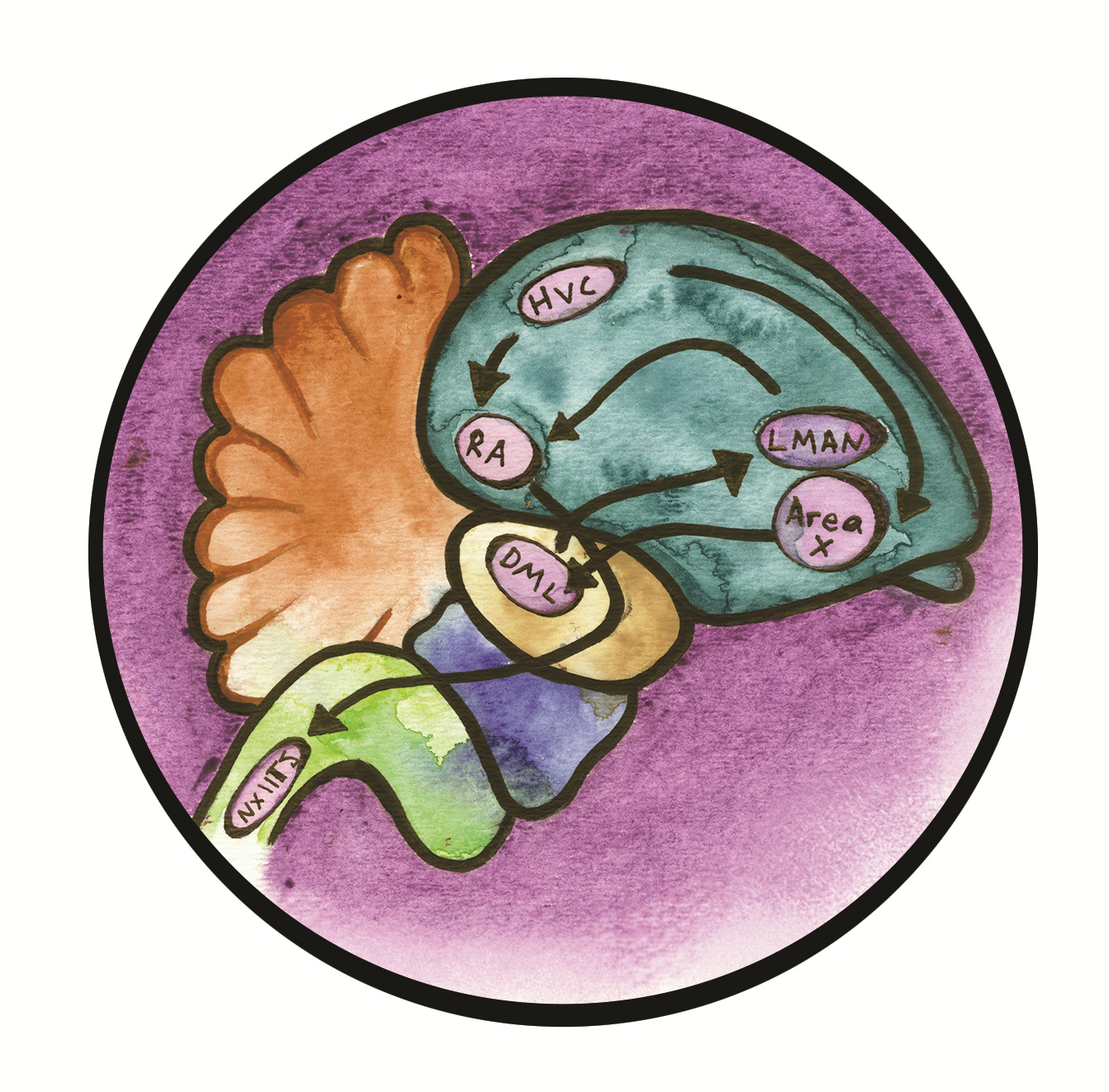
The pathway that is most relevant to this discussion is the “song learning,” or anterior fore brain pathway. This system passes information through the following song nuclei: first the HVC, directly to Area X, then to the DLM, next to the lMAN, and finally returning to Area X (Figure 1) [3]. Initially, the posterior descending pathway processes the sound that the songbird would like to imitate, then the sound is sent through the anterior forebrain pathway and travels through the song learning loop. The song is processed again in the nucleus V [4]. This information is then relayed to Area X, which stores a template of the song to be used as a source for comparison later. Next, this information is passed to the thalamus, which relays the signal to the lMAN, which acts as an editor for the song. This edited version is then passed back to Area X for comparison, where the song is further refined in the songbird’s learning. The information will be relayed back and forth from the lMAN to Area X until the song is perfected. Finally, when the song has been mastered, it is sent to the arcopallium (also known as the RA), located in the posterior descending pathway, which controls the motor movement of the muscles that enable song production [4]. Finally, the songbird produces song.
The sheer complexity of the songbird’s song system is understandable when we compare it to the human speech system—in fact, they are astoundingly similar. While the organization of the avian brain is quite distinct from the human brain, many of the structures are analogous in function [5]. For example, the avian brain region Area X is analogous to the human basal ganglia, which is responsible for reward learning, procedural learning, and language processing among many other functions. Meanwhile, the DLM is analogous to the human thalamus [2][5]. This conjures a pathway reminiscent of the mammalian pathway cortex of the basal ganglia to the thalamus and back to the cortex. Notably, the posterior pathway also parallels a human pathway that begins in the cerebral cortex and travels down the brainstem [2].
In addition, it has been noted that like human infants, young birds also undergo a stage of “babbling” when beginning to learn song [2]. This process—known as “subsong” in young birds—is analogous to the incoherent babbling of a baby, wherein the bird produces sounds that are variable with no noticeable pattern or repetition. A mature songbird will show characteristics of repetition, which can come in the form of imitation of its parent. Eventually the song becomes more stable and thus less variable [2]. Overall, these exceptional similarities between songbird and human speech learning suggest that, moving forward, learning about one system could be very important in learning the secrets of the other.
However, the songbird’s song learning system departs from human speech learning through the dopaminergic systems that reward and encourage singing [6]. This system is indicated by a dopamine release into Area X [7]. Interestingly, the strength of the dopamine release has been shown to vary based on the type of song being produced. If a bird is singing to impress a sexual partner, the dopamine level is higher than when a bird sings indirectly. Yet, it is notable that Area X still receives dopamine, though in lower amounts, even when birds sing for themselves. In contrast, while dopaminergic regulation has been shown to be important to speech control in speech impaired human subjects, there has been no study testing the specific mechanisms of dopaminergic regulation in healthy subjects [7].
The Secret to Speech
In comparison to the song system present in the songbird, the parrot has analogous neurological structures in vocal learning and production, with a few differences [8]. The significant difference between these two was unveiled through Chakraborty and colleagues’ research of another embedded song system within the parrot song system. This was done using the gene for parvalbumin (PVALB), which is a marker of song nuclei [9]. The researchers postulated that comparing the location of PVALB expression in the parrot brain with the location of its expression in songbirds could reveal brain structures that also contribute to parrot song. During their investigation, the researchers studied the expression of PVALB in the forebrain of a budgerigar parrot. They found that the budgerigar brain had areas of high PVALB gene expression indicative of a “core” song system and areas of moderate expression indicative of a “shell” song system that also included song nuclei. Thus, while the parrot song system deceivingly resembles the “core” song control system of songbirds at first glance, researchers found solely in parrots an additional system surrounding the “core” song system [8].
To ensure that this system was unique to the parrot, Chakraborty and colleagues examined serial sections of brain tissue in an Anna’s hummingbird and a zebra finch [7]. They found no shell song system in the samples. Since this secondary song system is only found in parrots, it is speculated that the shell song system may be the key—or at least one of the keys—to parrot speech mimicry. To affirm the consistency of the shell song system in parrots, the study also analyzed the brain structure of eight different parrots: the conure, the cockatiel, the yellow-lored amazon, the yellow-crowned amazon, the lovebird, the kea, the blue and gold macaw, and the African grey. Researchers discovered this secondary system in all of the aforementioned species [8].
The association of the shell system with vocal mimicry was strengthened by comparing brain tissue of these parrot species [8]. This comparison revealed variations in relative core and shell song system sizes. The budgerigars and the cockatiels were found to possess the largest core song systems; in contrast, the kea, the macaw and the African grey had the smallest relative core song systems. Notably, the parrots with the smaller relative core size are parrots with comparably higher vocal and cognitive abilities—thus hinting again to the shell song system’s likely role in speech mimicry [8].
In addition to this unique structure, Whitney and colleagues revealed another possible contributing factor to parrot vocal mimicry: the levels of FoxP2 expression in the brain [10]. Known for being involved in human speech development, FoxP2’s regulation in the parrot brain could indicate a similar role in parrots’ vocal development [10, 11]. The study used budgerigar parrots and a former study’s research on zebra finches (a songbird) to compare the natural levels of this gene during these species’ lifetimes and how they function accordingly [10]. In the previous study on zebra finches, results showed a trend toward downregulated FoxP2 during the zebra finches’ subsong phase, suggesting the gene plays a role in song practice. On the other hand, a downregulated trend was not found in the zebra finches’ adulthood, where song is non-plastic (non-changing) and directed toward mates. In contrast, Whitney and colleagues found that a trend toward downregulated FoxP2 was present in adult and juvenile budgerigars. Thus, this indicates that budgerigars are constantly in the period of subsong, or plastic song, and are able to learn new songs and sounds throughout their lives. In short, this comparison illustrates the continuous ability of the budgerigar to learn new songs, whereas the zebra finch only seems to have this ability in the early song learning stage until their learned practice song crystallizes when matured. Therefore, with current hypotheses regarding the expression of these genes, the unique ability of parrots to produce speech could very well be influenced by their plastic song learning. This plasticity not only opens up the possibility for parrots to learn sounds beyond their song developing stage but also suggests parrot song learning is not strictly associated with attracting mates, which is the case with most songbirds [10].
While parrots have additions to their song learning system, the songbird auditory system discourages heterospecific (different species) song learning [12]. Woolley and colleagues discussed this in their research concerning zebra finch conspecific (same species) vocal discrimination. Their results showed that auditory neurons in the zebra finch’s brain filtered out any extraneous sounds occurring in the natural environment, preventing these sounds from reaching the finch’s song learning system. The finch brain was found to best respond to songs that matched conspecific song; thus, the ability to mimic human speech, which does not resemble zebra finch song in the slightest, is not even a possibility since it is dismissed as extraneous noise. In contrast, the parrot most likely does not have homologous auditory song filters, allowing the parrot to expand its song repertoire beyond same-species song [12].
Why Speech?
While the parrot’s specialized neurological structures allow it to perform exceptional vocal feats, it is important to question how and why these structures evolved. Special cases of heterospecific mimicry in songbirds, such as the mynah bird, the mockingbird, the bowerbird, and eastern towhees, can offer some insight. Such studies have led researchers to form a variety of hypotheses on the evolutionary purpose of mimicry [13]. One such hypothesis, the Beau Geste hypothesis, suggests that the mimicking of other species could drive predators away from the mimicker’s territory. Using a large array of songs could give a predator the impression that a territory is diversely occupied and, as a result, steer them away [13]. While offering a legitimate reason for evolutionary mimicry, when applied to parrots, this theory makes little sense since the ability to produce more coherent mimicry tends to be correlated with greater parrot size; the African grey and the blue and gold macaw tend to have a more advanced mimicking repertoire in relation to smaller-sized lovebirds and budgerigars. This size correlation would be nonsensical when arguing for mimicry as a defense against predators because larger birds are less targeted than smaller ones. This is also significant when considering another rare, but notable, case of mimicking bird: the raven. The raven has few predators and yet has the ability to mimic human speech. On the other hand, parrots’ curious affinity for vocal learning could perhaps be connected to the dopamine release associated with learning birdsong. Similar to how the song system of singing provides songbirds with tiny bursts of pleasure, exploring different sounds could also be a way of pursuing a dopamine release [5]. As discussed above, the dopaminergic release is higher in songbirds during sexual display and lower when singing for no apparent reason; it is noteworthy that a dopamine release is still present in undirected singing or vocalizing. It is logical that the parrot could be similarly gleaning pleasure from its vocal performances.
Vocal learning has also been theorized to be an advantage among parrots when promoting social coherence in the flock [8]. Through this ability to mimic conspecific song, vocal learners can possibly facilitate information efficiently through a more elegant and advanced system [8]. This push to communicate and socialize with a flock was further discussed by Nottebaum in his observations of orange-winged amazon parrots [14]. These parrots developed dialects unique to their flock, and the dialect heard from flocks of the same species occupying other habitats sounded as different as two distinct species. Thus, in a situation where a parrot is only interacting with a human, the parrot’s dialect could begin to meld into the human’s due to its perception of the human as a flockmate [14].
Conclusion
The parrot brain is still an anomaly today. However, we can understand the basis of parrot brain structure by studying songbird song systems alongside the parrot-specific shell song systems and the accompanying studies in gene expression. While FoxP2 expression and shell song systems seem to hint at how the parrot brain is able to mimic human speech, not everything is entirely clear, in particular how human speech has developed in these birds. Many more studies must be conducted in order to further understand the parrot. For example, studies examining auditory song filters in the parrot brain could be useful in indicating if the lack of these song filters play a role in parrot speech. Additionally, researching the connection between parrot intelligence and vocal mimicry could reveal a link to this ability in parrots since some of the world’s most intelligent birds seem to possess the ability to mimic human speech—notably, the raven, which has no shell song system. Generally, more research into parrot speech can answer questions not only about this incredible ability, but also related questions about human speech and its development.
References
- “Quantifying the Amazing Diversity of Life: How Many Animals Are in the World?” WAF, 30 Nov. 2023, worldanimalfoundation.org/advocate/how-many-animals-are-in-the-world/.
- Nottebohm, F. (2005). The Neural Basis of Birdsong. PLoS Biology,3(5). doi:10.1371/journal.pbio.0030164
- Jarvis, E. D. (2004). Learned Birdsong and the Neurobiology of Human Language. Annals of the New York Academy of Sciences,1016(1), 749-777. doi:10.1196/annals.1298.038
- Suthers, R. A. (2004). How birds sing and why it matters. Natures Music,272-295. doi:10.1016/b978-012473070-0/50012-8
- Bolhuis, J. J., Okanoya, K., & Scharff, C. (2010). Twitter evolution: Converging mechanisms in birdsong and human speech. Nature Reviews Neuroscience,11(11), 747-759. doi:10.1038/nrn2931
- Kubikova, Ľ, & Košťál, Ľ. (2010). Dopaminergic system in birdsong learning and maintenance. Journal of Chemical Neuroanatomy,39(2), 112-123. doi:10.1016/j.jchemneu.2009.10.004
- Simonyan, K., Horwitz, B., & Jarvis, E. D. (2012). Dopamine regulation of human speech and bird song: A critical review. Brain and Language,122(3), 142-150. doi:10.1016/j.bandl.2011.12.009
- Chakraborty, M., Walløe, S., Nedergaard, S., Fridel, E. E., Dabelsteen, T., Pakkenberg, B., . . . Jarvis, E. D. (2015). Core and Shell Song Systems Unique to the Parrot Brain. Plos One,10(6). doi:10.1371/journal.pone.0118496
- Hara, E., Rivas, M. V., Ward, J. M., Okanoya, K., & Jarvis, E. D. (2012). Convergent Differential Regulation of Parvalbumin in the Brains of Vocal Learners. PLoS ONE,7(1). doi:10.1371/journal.pone.0029457
- Whitney, O., Voyles, T., Hara, E., Chen, Q., White, S. A., & Wright, T. F. (2014). Differential FoxP2 and FoxP1 expression in a vocal learning nucleus of the developing budgerigar. Developmental Neurobiology,75(7), 778-790. doi:10.1002/dneu.22247
- Lai, C. S., Fisher, S. E., Hurst, J. A., Vargha-Khadem, F., & Monaco, A. P. (2001). A forkhead-domain gene is mutated in a severe speech and language disorder. Nature,413(6855), 519-523. doi:10.1038/35097076
- Woolley, S. M., Fremouw, T. E., Hsu, A., & Theunissen, F. E. (2005). Tuning for spectro-temporal modulations as a mechanism for auditory discrimination of natural sounds. Nature Neuroscience,8(10), 1371-1379. doi:10.1038/nn1536
- Kelley, L. A., Coe, R. L., Madden, J. R., & Healy, S. D. (2008). Vocal mimicry in songbirds. Animal Behaviour,76(3), 521-528. doi:10.1016/j.anbehav.2008.04.012
- Pepperberg, I. M. (2002). The Alex studies: Cognitive and communicative abilities of grey parrots. Cambridge, MA: Harvard University Press.
