Imagine sitting down with a doctor to discuss several distressing symptoms you’ve been experiencing recently. Slowly you confess that you’ve been experiencing a sense of numbness, and your hobbies don’t interest you. In fact, nothing interests you anymore. Simple tasks feel impossible. You can’t wrap your head around what’s wrong. She carefully explains that you are suffering from depression and that antidepressants would be a good course of action for you. As the weeks go by and you take your prescription, you begin to notice significant changes in your mood. A physical weight that was once nearly crushing has become bearable and manageable. You are now able to perform simple daily tasks like getting out of bed, and you also even find the energy to socialize with friends. The world feels clearer and brighter. Overall, you experience a general sense of well-being. However, soon you begin to notice the previously reduced symptoms are suddenly returning with full force. Things begin to spin out of control and there doesn’t seem to be a cause for this confusing, rapid decline in your mental health. Your life has not changed dramatically; you have taken your medication at every prescribed interval, and you have not done anything the doctor warned you away from, such as drinking alcohol or using illicit drugs while taking your medication. This may seem like an unexpected and frustrating experience; however, this is the reality for many people. While researchers are still trying to answer the questions of what goes wrong in these cases and why a pharmacological phenomenon known as tachyphylaxis could be the explanation.
What is Tachyphylaxis?
Tachyphylaxis (ta-kuh-fuh-lak-suhs) describes the progressive and rapid decrease in drug effectiveness as well as the re-emergence of symptoms in response to the administration of medications [1]. Sometimes the efficacy of a drug unexpectedly and suddenly decreases, even after the body has previously only received one dose. A few possible causes for this condition relate to receptor desensitization, changes in larger neural pathways linked to GABA and serotonin, and loss of the placebo effect. We will explore some of these mechanisms in depth later in the article. While tachyphylaxis may sound the same as tolerance, another phenomenon in which drug efficacy changes for an individual taking the drug over a long period of time, the two have marked differences. For instance, tachyphylaxis corresponds to an acute decrease in drug efficacy, rather than the slow decline over subsequent doses that characterizes tolerance [2]. Another key difference is in how modifying the dose will alter the medication’s effect. If someone is experiencing tolerance, they may take a heightened dose to produce the same effect: think of drugs like alcohol or opioids. On the other hand, tachyphylaxis is not dose-dependent and a larger dose may not restore the desired effect [3]. A variety of drugs exhibit differing degrees of tachyphylaxis, including treatments for asthma and corticosteroids. However, as the majority of tachyphylaxis research focuses on the treatment of Major Depressive Disorder (MDD) with antidepressants, tachyphylaxis specific to MDD will be the focus of the article. In regards to antidepressants, tachyphylaxis is specifically characterized as a return of depressive symptoms following a full recovery from a major depressive episode despite the fact that previously effective medications are still being taken. Some of these relapses can astoundingly happen within 6 months [1]. But first, for tachyphylaxis to occur, someone must be formally diagnosed with depression.
The Basics of Depression
A diagnosis of depression requires a person to meet a formal set of criteria used by psychologists. The Diagnostic and Statistical Manual of Mental Disorders (DSM-5), a manual that extensively outlines and categorizes recognized mental disorders, outlines nine different symptoms that are commonly found in individuals with MDD [4]. A person with MDD is defined by the manual as someone who experiences five or more of the symptoms listed during a consecutive two-week period [4]. These symptoms include but are not limited to a depressed mood for most of the day, diminished interest or pleasure in all or most activities, weight loss or weight gain, fatigue, and feelings of worthlessness. While a combination of any five symptoms indicates a person may have MDD, for patients with tachyphylaxis, no symptom has been documented as returning more frequently or faster than another. Rather, a general return of some or all of the original symptoms helps doctors and researchers identify tachyphylaxis.
Throughout the years, both inherent and environmental risk factors have been documented for depression. Environmental factors are often the primary focus for determining the cause of depression because they are relatively easy to identify. For example, one environmental risk factor for developing depression is stress [5]. Other environmental risk factors relate to our lifestyle choices: diet, exercise, and sleep are three such influences that play a significant role in developing depression [6][7][8][9].
Even so, those who live in nurturing, stable, and healthy environments can develop depression. This is due to the presence of unseen influences for developing depression, known as inherent risk factors; they reside within us without a definitive external trigger or cause. One of the most well documented risk factors is cognitive vulnerability, a pattern of thinking or cognitive bias that predisposes a person to psychological problems [10]. Biases in attention, memory, as well as repetitive negative thoughts, are common across emotional disorders such as depression [11]. However, the cause of these biases themselves is unknown. Recent studies suggest that parental history of depression could be a causal mechanism contributing to negative biases in thinking [12]. For example, a study by researchers at Stanford University showed that female individuals at risk for developing depression due to their mother’s mental health issues can be characterized by specific cognitive biases. Such cognitive biases were deeply ingrained negative patterns of thinking, activated by negative events, and could potentially be genetically passed from mother to daughter. The researchers hypothesized that high-risk youth could acquire cognitive biases through their frequent exposure to disordered biases displayed in their disordered parent [12].
Some inherent risk factors can also make it harder to recover from depression. Imagine you and your friend go through a particularly intense traumatic event. You have the same family histories of emotional disorders as well as similar home lives, and after experiencing a shared trauma, you both are later diagnosed with depression. Through therapy and support, your friend is able to recover from depression. But even though you follow the same exact steps as her, you’re still stuck in the rut of your own depression. What could be inherently different between you and your friend? One possible explanation for this phenomenon is known as the “serotonin hypothesis”. The serotonin hypothesis proposes that diminished serotonin-driven activity is linked to the changes in physiological processes that characterize depression, the mechanism of which has been well-documented by studies on tryptophan depletion. Researchers studied tryptophan, a necessary amino acid for serotonin manufacturing, in a group of participants that are divided into two groups: one with risk factors for depression and the other without [13]. In both groups, some received no treatment, and some had their tryptophan levels experimentally reduced via an acute dietary change. Researchers found that participants who had previous clinical bouts of depression exhibited depressive symptoms upon their tryptophan levels being decreased, while those who did not have risk factors showed no symptoms. Even more surprisingly, people who had risk factors for depression, like family history, showed fewer symptoms than the participants with previous clinical bouts of depression [13]. If impaired serotonin function can prevent recovery from depression and cause more subsequent depressive episodes, there is a great incentive in finding a remedy for it. This is why serotonin function and its pathways are a strong point of interest when creating antidepressants. But what exactly does serotonin do in the brain, and how do antidepressants affect this pathway?
Serotonin’s Voyage through the Brain and Antidepressants
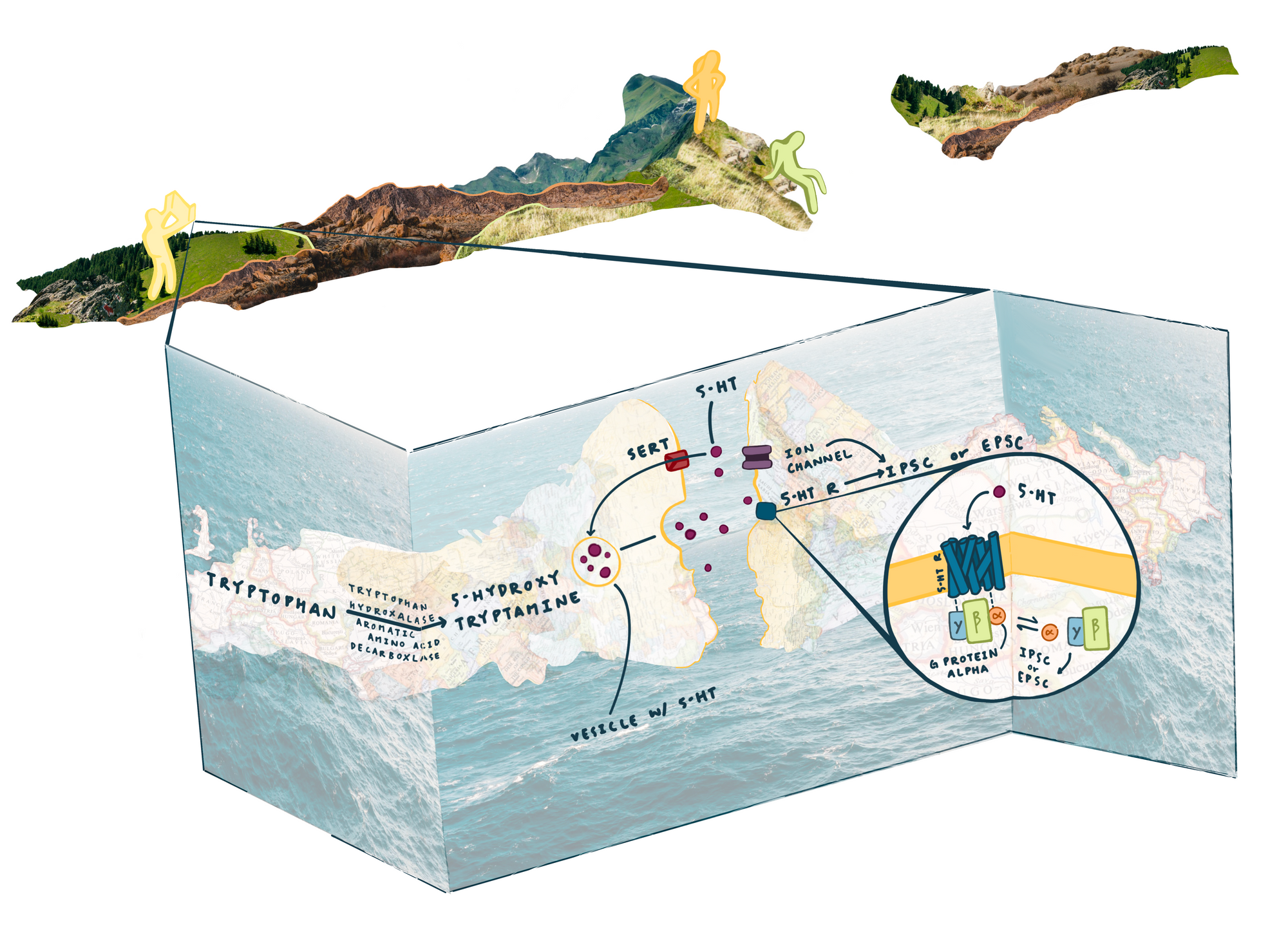
Serotonin, sometimes known as 5-hydroxytryptamine, or 5-HT, is involved in appetite, mood regulation, sexual desire, sleep, and social behavior [14]. It is classified as a neurotransmitter and is synthesized in the gut as well as in the central nervous system. Serotonin receptors are proteins that can respond to serotonin and transmit signals to sensory nerves, and they are grouped into seven main families: 5-HT1, 5-HT2, 5-HT3, 5-HT4, 5-HT5, 5-HT6, 5-HT7. All but 5-HT3 are G protein-coupled receptors (GPCRs) [14]. Let’s take a trip into the brain and learn about the physiology of the synapse, as well as GPCRs, so we can better imagine how serotonin executes its role in the brain and how medications affect this process.
If we could become microscopic and enter the brain, we would notice very small gaps between neurons, known as synaptic clefts, across which neurons send signals to each other. Before anything can happen in the synaptic cleft, a signal called the action potential must propagate down the presynaptic neuron to the axon terminal, the end of the neuron. The signal reverberates to little packets holding neurotransmitters called vesicles. The vesicles will travel to the cell membrane and release the neurotransmitter (in this case, serotonin) into the synaptic cleft to communicate the action potential to other neurons. Different amounts of the neurotransmitter will be released depending on how frequent the action potentials are, as more or less vesicles will release their contents into the cleft. Now, the free floating neurotransmitters travel across the cleft and slot into the minuscule chambers of the postsynaptic neuron, called receptors [15][16].
Some neurotransmitters help to prevent an action potential from being transmitted from one neuron to the next, while others help propagate it forward. These are known as inhibitory and excitatory signals, respectively. There is a quite astounding number of combinations that create excitatory or inhibitory signals [17]. Some neurotransmitters have a strong affinity for only inhibitory or excitatory receptors, while others may bind to both excitatory or inhibitory receptors, attaching to whichever type is on postsynapse. The ability to create or prevent an action potential is dependent on changes in specific ion concentrations inside and outside the neuron and how many of them can be transported through channels in the membrane. Ions can be positively or negatively charged, and what direction that charge moves in, either inside or outside of the post-synaptic cell, can change the probability of action potential propagation. Excitatory receptors prompt the flow of positive charge into the neuron and can trigger action potentials. Conversely, inhibitory receptors prevent or reduce flow of positive charge and block action potentials. G protein coupled receptors (GPCRs) are one way to help regulate this ion flow process [18].
First, an external signaling molecule (serotonin) binds to the GPCR 5-HT receptor. Serotonin causes a conformational, or shape, change on the 5-HT receptor. The GPCR and its subunits interact with other molecules in the postsynapse to create the appropriate cellular response. Many different types of responses can occur, but all of them contribute to the desired effect of the serotonin neurotransmitter. For example, some GPCRs are able to regulate excitatory and inhibitory currents by mediating the flow of ions into the postsynapse, like the calcium ion [19]. Now that the serotonin has completed its job, serotonin pops out of the receptor and flows back into the presynapse through the serotoninergic transporter (SERT).
For treating depression, there are many kinds of medications. Most prescribed medications fall into the category of selective serotonin reuptake inhibitors (SSRIs). There are three basic neurotransmitters that researchers have linked to depression: dopamine, norepinephrine, and serotonin [20]. Recall serotonin from our intercellular journey above. SSRIs function by increasing the amount of time the serotonin is able to influence and affect the brain, combatting the effects proposed in the serotonin hypothesis. When serotonin receptors are depleted or damaged, fewer serotonin molecules are able to interact with the receptors and create signals. This results in less of the signals serotonin would normally cause, and can produce symptoms of depression and/or prevent recovery. However, if an SSRI swoops in, it can combat this unpleasant cycle. SSRIs work to prevent the reuptake and reuse of serotonin done by our little friend, SERT, so SERT can no longer transport serotonin back into the presynapse as quickly [21]. Serotonin can now inhabit the synaptic cleft longer. If serotonin is in the cleft, it may interact with serotonin GPCR receptors for longer before it is taken back into the presynapse. This results in a larger and stronger cellular signal. Researchers have hypothesized that the stronger signal helps by aiding the processing of emotionally-laden information at a subconscious level and positively shifting automatic emotional responses [13].

Tachyphylaxis and Antidepressants
Tachyphylaxis can rapidly nullify the positive effects of SSRIs. In a review of relevant clinical trials, rates of tachyphylaxis, which were measured by evaluating symptoms of depression and their severity, varied from 9% to 57% for those taking antidepressants, which is a staggering range [22]. These reports have been supported by a study conducted by researchers at the University of Pennsylvania School of Medicine [23]. They treated 276 patients with 150-200mg of Sertraline, a common SSRI, and found that every 8 weeks, the efficacy of the drug dropped by 19.9%. This means more previous drug exposure was correlated with lowered efficacy of the drugs [28]. Also, those who experience antidepressant tachyphylaxis may be less responsive to new treatment interventions [24]. In other words, it may be difficult for those who have experienced tachyphylaxis to simply switch to another antidepressant and experience the same benefits as the previous one. This is also quite troubling because those who are taking antidepressants and experience tachyphylaxis may begin to feel the urge to self-harm or experience thoughts of suicide without effective treatment options [25]. All of the combined factors listed above elucidate that tachyphylaxis is a serious issue that needs to be addressed.
Finding treatments for tachyphylaxis is extremely difficult because the mechanisms of tachyphylaxis are largely unknown. However, we have some biochemical clues for why tolerance occurs on the cellular level in neurons. Tolerance begins when, over time, after a substance has been constantly binding with the receptor, the receptor becomes desensitized [26]. It can also be caused by a smaller quantity of the substance reaching its destination because more enzymes that break down the substance are being created in response to repeated use [27]. Other reasons for tolerance have been found as well, and they are mostly well-documented. Tachyphylaxis, however, is another story. Our understanding of tolerance is like a poorly lit hotel room. The room is not particularly well lit, but you can see most of the objects and understand them as well. However, tachyphylaxis is similar to being in the same hotel room in a horror video game. You have a dim flashlight that runs out of battery constantly. The once comprehensible objects are foreign and hard to make out or understand. The items in the room are akin to mechanisms and causations of tolerance and tachyphylaxis and the light is research, knowledge, and comprehension of similar mechanisms. Tachyphylaxis’s systems are difficult to understand because our “light” is lacking, but this does not mean that people are not trying to brighten that light and understand the biochemical reasoning of tachyphylaxis.
Dr. Katz from the Jerusalem Mental Health Center postulated some possible mechanisms, such as nonadherence, loss of the placebo-effect, receptor desensitization, and undiagnosed bipolar disorder [1]. Some doubt the existence of true tachyphylaxis and attribute the loss of antidepressant response to nonadherence and/or loss of placebo effect. However, this hypothesis cannot explain all factors related to tachyphylaxis, such as differences in genetic features playing a significant role in patients’ responses to antidepressants. Others theorize the true cause of tachyphylaxis relates to an improper diagnosis, not the type of medication. Antidepressant tachyphylaxis may occur more frequently in patients who have type II bipolar disorder compared to those who have depression, as antidepressants have been shown to worsen episodes of mania [28]. If a person was misdiagnosed with MDD instead of bipolar depression, which have some overlap in symptoms, they may be experiencing tachyphylaxis because the neurological mechanisms of the two disorders are different, and therefore require different treatments for effective management. There are dozens of other hypotheses aiming to explain the mechanisms of tachyphylaxis, however, we will take an in-depth look at receptor desensitization because this is what the large majority of current tachyphylaxis treatment research surrounds.
Some researchers believe that serotonin receptor desensitization is the primary cause for an increase in the return of depressive symptoms after long-term treatment of SSRIs. Even though the exact mechanisms are unknown, research suggests there may be a change in sensitivity and/or the number of cellular receptors, second messenger systems, or other systems related to receptor function [24]. One study, conducted at Vrije Universiteit Amsterdam, proposed that a change on a larger scale than SSRI desensitization could be the possible cause of tachyphylaxis [31]. Researchers created a social stress paradigm, where an intruder rat was introduced into the territory of a dominant male (the stressor) rat and allowed to fight. In addition to the time spent with the dominant rat, the intruder rat was also housed alone as another stressor. Over subsequent cycles of this, the intruder rats began to show signs of anhedonia. Formally, anhedonia is known as the inability to feel pleasure and is a key symptom of depression. In the study, the researchers assessed anhedonia by a measured absence of anticipatory behavior in response to sugar water rewards. After anhedonia was established, the intruder mice were treated with a common SSRI, fluoxetine. The goal of this treatment was to measure 5-HT and GABA(B) receptor functionality. GABA(B) receptors are receptors for the neurotransmitter gamma-aminobutyric acid (GABA), the main inhibitory neurotransmitter in the central nervous system [30]. GABA(B) was measured along with 5-HT(1A) in order to determine if the 5-HT(1A) receptor functionality change was exclusive to the 5-HT pathway, or if the GABA(B) pathway was also affected. After repeated administration of fluoxetine, there were signs of desensitization of the 5-HT(1A) receptors, but also signs of desensitization of GABA(B) receptors. Researchers think that this implies that SSRIs desensitize 5-HT receptors via a downstream pathway shared with GABA(B) [31]. While this study brings no definitive conclusions for tachyphylaxis, it is another piece of the puzzle to understanding the arduous problem.
Even with this promising research, the mystery still surrounding the exact causes of tachyphylaxis makes treatment frustratingly difficult for patients as well as healthcare providers. Some researchers suggest increasing the medication dosage may be useful to combat tachyphylaxis, while others suggest drug holidays in which a person stops taking their medication for a period of time, or decreases their current dose: two completely different propositions for the same problem [29]. There is some hope, though. Dual Reuptake Inhibitors (DRIs) or Serotonin Norepinephrine Reuptake Inhibitors (SNRIs) are types of antidepressants that inhibit reuptake in not only serotonin, but norepinephrine as well. This means that even if tachyphylaxis occurs to the serotonin reuptake inhibitors, norepinephrine reuptake inhibitors may still perform their role, or vice versa. SNRIs may help incur lower rates in relapse and recurrence of depressive symptoms. For example, during a trial, SSRI-induced tachyphylaxis was reduced from 14.1% to 3.7% when dual reuptake inhibitors were used in their place [32]. Additionally, scientists are looking somewhere seemingly strange: histamines. Usually when one thinks of histamines, or more likely, antihistamines, they imagine their friend whose nose mirrors a water feature at a water park: Suppressing allergy-induced reactions has thus far been the main purpose of antihistamines. However, recent research could have us looking to expand our definition. Scientists are tapping into histamine and its receptors to unlock new insights into psychiatric conditions including but not limited to: depression, obsessive-compulsive disorder, and schizophrenia. Some already-approved antidepressants interact with the H1 receptor, a type of histamine receptor found in the central nervous system [33]. Additionally, recent experiments in mice have revealed that histamine-related inflammation can influence mood-stabilizing neurotransmitters like serotonin. Researchers believe further study of how all of these transmitters and receptors work together could improve serotonin treatments for depression in humans [34].
Other Treatments for Depression
Besides medications, there are many other treatments for depression. Psychological therapy for depression displays powerful capabilities in creating successful long-term outcomes [35]. There are numerous different types of established therapy methods, as well as new ones that are being tested for their efficacy. One established type of therapy is known as cognitive behavioral therapy (CBT). CBT is based upon the step-by-step guidebook Cognitive Therapy: Basics and Beyond written by Judith Beck [36]. It has been proven as an effective method aimed at changing maladaptive emotional responses by creating skills to change associated thoughts and/or behaviors [37]. It is usually brief, goal-oriented, and works to address specific concerns [38]. Another effective and common type of therapy is called interpersonal therapy (IPT). Here the focus is on stressful life events of grief, interpersonal disputes, life transitions, and/or social isolation or deficits that are linked to or caused by current symptoms (such as depression). IPT’s goal is to help a patient connect with social supports and improve their quality of relationships [35]. Certain therapy styles are not mutually exclusive and can be used interchangeably depending on the current state of the patient and the exherent struggles they may be facing. Most treatments of depression include a combination of prescription antidepressants and therapy [39]. That’s why when tachyphylaxis occurs in a patient, the simple alternative is not merely to switch to therapy. Most likely, they are already attending therapy in some form in combination with the medications that previously helped.
Conclusion
Treating mental illness seems almost as difficult as understanding mental illness itself. However daunting as the challenge may be, the importance of finding solutions has become even more pressing in the modern world. The World Health Organization states that the number of people living with depression worldwide increased by 18.4% between 2005 and 2015 [40]. These frightening numbers should not deter us from the important task at hand, but rather encourage us. Our problems have yet to be solved because the human mind is so complex, but this is a double edged sword. On one hand, because of the mind’s complicated nature, it is astoundingly difficult to fully understand it. On the other hand, the complexity of the human mind is what makes us capable of solving such formidable puzzles. Solving problems, uncovering secrets, and discovering how the world works is what makes the world of science so unique and fascinating. With continued research, people who continue to struggle with tachyphylaxis may one day find a definitive answer to this problem.
Resources
- Katz G. (2011). Tachyphylaxis/ tolerance to antidepressive medications: a review. The Israel journal of psychiatry and related sciences, 48(2), 129–135.
- Katzung BG, ed. Basics of Clinical Pharmacology, 8th ed. London: McGraw/Hill. 2001.
- Vashishta R, & Berrigan M.J. (2014). Drug tolerance and tachyphylaxis. Freeman B.S., & Berger J.S.(Eds.), Anesthesiology Core Review: Part One Basic Exam. McGraw Hill. https://accessanesthesiology.mhmedical.com/Content.aspx?bookid=974§ionid=61587630
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM-5), Fifth edition. 2013.
- Brown GW, Harris TO, eds. 1989. Life Events and Illness. New York: Guilford Press
- Baglioni, C., Battagliese, G., Feige, B., Spiegelhalder, K., Nissen, C., Voderholzer, U., Lombardo, C., & Riemann, D. (2011). Insomnia as a predictor of depression: a meta-analytic evaluation of longitudinal epidemiological studies. Journal of affective disorders, 135(1-3), 10–19. https://doi.org/10.1016/j.jad.2011.01.011
- Jacka, F. N., Pasco, J. A., Mykletun, A., Williams, L. J., Hodge, A. M., O'Reilly, S. L., Nicholson, G. C., Kotowicz, M. A., & Berk, M. (2010). Association of Western and traditional diets with depression and anxiety in women. The American journal of psychiatry, 167(3), 305–311.
- Lopresti, A. L., Hood, S. D., & Drummond, P. D. (2013). A review of lifestyle factors that contribute to important pathways associated with major depression: diet, sleep and exercise. Journal of affective disorders, 148(1), 12–27. https://doi.org/10.1016/j.jad.2013.01.014
- Sánchez-Villegas, A., Toledo, E., de Irala, J., Ruiz-Canela, M., Pla-Vidal, J., & Martínez-González, M. A. (2012). Fast-food and commercial baked goods consumption and the risk of depression. Public health nutrition, 15(3), 424–432. https://doi.org/10.1017/S1368980011001856
- Riskind, John H.; Black, David (2005). "Cognitive Vulnerability". In Freeman, Arthur; Felgoise, Stephanie H.; et al. (eds.). Encyclopedia of Cognitive Behavior Therapy. New York: Springer. pp. 122–26. ISBN 9781429411738.
- Mathews, A., & MacLeod, C. (2005). Cognitive vulnerability to emotional disorders. Annual review of clinical psychology, 1, 167–195. https://doi.org/10.1146/annurev.clinpsy.1.102803.143916
- Dearing KF, Gotlib IH. 2009. Interpretation of ambiguous information in girls at risk for depression. J. Abnorm. Child Psychol. 37(1):79–91
- Cowen, P. J., & Browning, M. (2015). What has serotonin to do with depression?. World psychiatry : official journal of the World Psychiatric Association (WPA), 14(2), 158–160. https://doi.org/10.1002/wps.20229
- Alberts B, Johnson A, Lewis J, Raff M, Roberts K, Walkter P (2008) Molecular Biology Of The Cell, 5th edition, New York: Garland Science. 676, 683.
- Mochida, S. (2015). Presynaptic terminals. Springer.
- David, D. J., & Gardier, A. M. (2016). Les bases de pharmacologie fondamentale du système sérotoninergique : application à la réponse antidépressive [The pharmacological basis of the serotonin system: Application to antidepressant response]. L'Encephale, 42(3), 255–263. https://doi.org/10.1016/j.encep.2016.03.012
- Purves D, Augustine GJ, Fitzpatrick D, et al., editors. Neuroscience. 2nd edition. Sunderland (MA): Sinauer Associates; 2001. Excitatory and Inhibitory Postsynaptic Potentials. Available from: https://www.ncbi.nlm.nih.gov/books/NBK11117/
- Hyman S. E. (2005). Neurotransmitters. Current biology : CB, 15(5), R154–R158. https://doi.org/10.1016/j.cub.2005.02.037
- Nutt D. J. (2008). Relationship of neurotransmitters to the symptoms of major depressive disorder. The Journal of clinical psychiatry, 69 Suppl E1, 4–7.
- Huang, Y., & Thathiah, A. (2015). Regulation of neuronal communication by G protein-coupled receptors. FEBS letters, 589(14), 1607–1619. https://doi.org/10.1016/j.febslet.2015.05.007
- Zhou, Z., Zhen, J., Karpowich, N. K., Law, C. J., Reith, M. E., & Wang, D. N. (2009). Antidepressant specificity of serotonin transporter suggested by three LeuT-SSRI structures. Nature structural & molecular biology, 16(6), 652–657.
- Kinrys, G., Gold, A. K., Pisano, V. D., Freeman, M. P., Papakostas, G. I., Mischoulon, D., Nierenberg, A. A., & Fava, M. (2019). Tachyphylaxis in major depressive disorder: A review of the current state of research. Journal of affective disorders, 245, 488–497. https://doi.org/10.1016/j.jad.2018.10.357
- Amsterdam, J. D., Williams, D., Michelson, D., Adler, L. A., Dunner, D. L., Nierenberg, A. A., Reimherr, F. W., & Schatzberg, A. F. (2009). Tachyphylaxis after repeated antidepressant drug exposure in patients with recurrent major depressive disorder. Neuropsychobiology, 59(4), 227–233. https://doi.org/10.1159/000226611
- Targum S. D. (2014). Identification and treatment of antidepressant tachyphylaxis. Innovations in clinical neuroscience, 11(3-4), 24–28.
- Schimelpfening, N. (n.d.). What to do if your antidepressant stops working. Verywell Mind. Retrieved October 28, 2021, from https://www.verywellmind.com/what-to-do-if-your-antidepressant-has-stopped-working-1066864.
- Bespalov, A., Müller, R., Relo, A. L., & Hudzik, T. (2016). Drug Tolerance: A Known Unknown in Translational Neuroscience. Trends in pharmacological sciences, 37(5), 364–378. https://doi.org/10.1016/j.tips.2016.01.008
- Cederbaum A. I. (2012). Alcohol metabolism. Clinics in liver disease, 16(4), 667–685. https://doi.org/10.1016/j.cld.2012.08.002
- Amsterdam, J. D., & Shults, J. (2009). Does tachyphylaxis occur after repeated antidepressant exposure in patients with Bipolar II major depressive episode?. Journal of affective disorders, 115(1-2), 234–240. https://doi.org/10.1016/j.jad.2008.07.007
- Byrne, S. E., & Rothschild, A. J. (1998). Loss of antidepressant efficacy during maintenance therapy: possible mechanisms and treatments. The Journal of clinical psychiatry, 59(6), 279–288. https://doi.org/10.4088/jcp.v59n0602
- Pinard, A., Seddik, R., & Bettler, B. (2010). GABAB receptors: physiological functions and mechanisms of diversity. Advances in pharmacology (San Diego, Calif.), 58, 231–255. https://doi.org/10.1016/S1054-3589(10)58010-4
- Cornelisse, L. N., Van der Harst, J. E., Lodder, J. C., Baarendse, P. J., Timmerman, A. J., Mansvelder, H. D., Spruijt, B. M., & Brussaard, A. B. (2007). Reduced 5-HT1A- and GABAB receptor function in dorsal raphé neurons upon chronic fluoxetine treatment of socially stressed rats. Journal of neurophysiology, 98(1), 196–204. https://doi.org/10.1152/jn.00109.2007
- Posternak, M. A., & Zimmerman, M. (2005). Dual reuptake inhibitors incur lower rates of tachyphylaxis than selective serotonin reuptake inhibitors: a retrospective study. The Journal of clinical psychiatry, 66(6), 705–707. https://doi.org/10.4088/jcp.v66n0605
- Comas-Basté, O., Sánchez-Pérez, S., Veciana-Nogués, M. T., Latorre-Moratalla, M., & Vidal-Carou, M. (2020). Histamine Intolerance: The Current State of the Art. Biomolecules, 10(8), 1181. https://doi.org/10.3390/biom10081181
- Jablonowski, J. A., Carruthers, N. I., & Thurmond, R. L. (2004). The histamine H4 receptor and potential therapeutic uses for H4 ligands. Mini reviews in medicinal chemistry, 4(9), 993–1000. https://doi.org/10.2174/1389557043403152
- Cuijpers, P., Donker, T., Weissman, M. M., Ravitz, P., & Cristea, I. A. (2016). Interpersonal Psychotherapy for Mental Health Problems: A Comprehensive Meta-Analysis. The American journal of psychiatry, 173(7), 680–687. https://doi.org/10.1176/appi.ajp.2015.15091141
- Beck, J. S. (2011). Cognitive behavior therapy: Basics and beyond (2nd ed.). Guilford Press.
- Kaczkurkin, A. N., & Foa, E. B. (2015). Cognitive-behavioral therapy for anxiety disorders: an update on the empirical evidence. Dialogues in clinical neuroscience, 17(3), 337–346. https://doi.org/10.31887/DCNS.2015.17.3/akaczkurkin
- Fenn, K., & Byrne, M. (2013). The key principles of cognitive behavioural therapy. InnovAiT: Education and Inspiration for General Practice, 6(9), 579–585. https://doi.org/10.1177/1755738012471029
- Tiller J. W. (2013). Depression and anxiety. The Medical journal of Australia, 199(S6), S28–S31. https://doi.org/10.5694/mja12.10628
- Depression and Other Common Mental Disorders: Global Health Estimates. Geneva: World Health Organization; 2017. License: CC BY-NC-SA 3.0 IGO.
