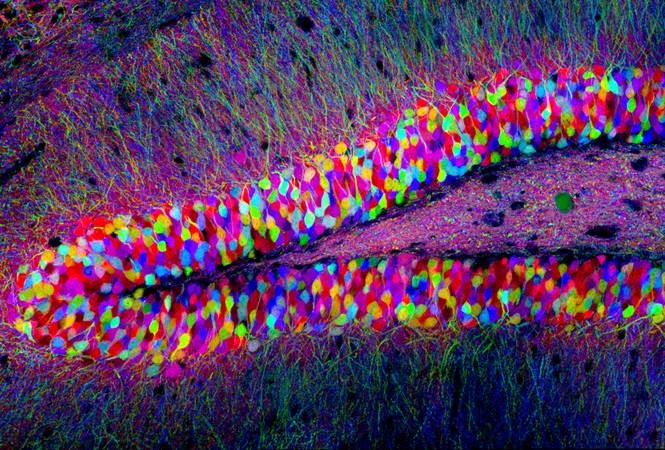
Image Credit: Tamily Weissman, Harvard
Those who have been reading carefully these past few days may have noticed the emergence of a theme in my reports. After starting with the way the tissue in the midbrain responds to changes in cellular and molecular signals , and continuing with an insight into how memories are stored and perturbed via protein synthesis and disruption of the local environment involved in such memory retention, it became evident that a distinct interplay between cells, their constituents, and the environment was at work, dictating the way in which physical, neural alterations were made.
Then, by examining the ways we study neuronal circuitry through a “Connectomic” approach, it was made clear that a different type of neural activity, not regulated by genetic and molecular factors, but by connectivity and experience, also shapes brain function.
When considered together, these concepts illustrate the brain’s immense and multifaceted ability to interact with the environment and undergo changes in response to the demands imposed upon it. There is another topic I will briefly consider here that suggests a mechanism for how the brain is able to translate adjustments at the microscopic level into actual behavioral responses.
Neuroplasticity is the term given to describe the brain’s capacity to change. To understand neuroplasticity it is helpful to imagine a calculator. In this device, no matter how many times you input 1+1, the answer will always be 2, and the time it takes to reach that answer will always stay the same. In the brain, however, repeated activation of a particular circuit or synapse will alter that circuit or synapses’ ability to function, by increasing its sensitivity, for example. The brain, unlike a calculator, can respond faster over time and alter its output. Synapses that are not activated, however, may undergo “pruning”, in which case they die.
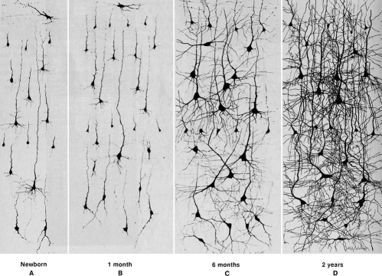
Neurogenesis during early development.
Image credit: Courchesne et al., 2007
The brain is most noticeably plastic during times of development as new neurons are being born and circuits are undergoing alterations at a high frequency. There is however, an emerging focus on plasticity in the adult brain. Hongjun Song’s presentation on adult neurogenesis provided a very interesting look into some of the mechanisms guiding this process.
Evidence has been provided showing adult neurogenesis in multiple areas of the brain – one being the hippocampus, which according to Spalding et. al., can generate 700 new neurons per day [1] . Song decided to focus his research here, asking the question: how does this neurogenesis take place, and how do newborn neurons behave in a way that allows them to function differently than preexisting neurons?
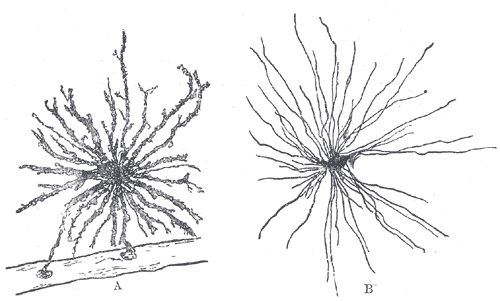
Radioglial cells
Working their way through different theories, researchers were able to show that the process of neurogenesis was controlled by a specific type of neural cell, a radioglial cell, acting as a multipotent stem cell. After developing a brain specific technique for tracing the lineage of cells, it was discovered that radioglial cells exhibited the ability to differentiate into either neurons, astrocytes, or more radioglia depending on the symmetry of the parent radioglial cell’s division. Asymmetric division yielded neurons and astrocytes while symmetric division produced more radioglia.
With an idea of how the process of neurogenesis occurred, researchers went on to study the behavior of these new neurons. Perhaps not surprisingly, it was found that these neurons behaved similarly to embryonic neurons, and when studies testing their ability to undergo long term potentiation were conducted, the newborns outperformed established populations, showing a higher amplitude postsynaptic response to input and a lower threshold for long term potentiation induction [2] .
This finding points toward the role neurogenesis might play in neuroplasticity, highlighting newborn neurons’ part in mediating learning, memory acquisition, and strengthening of the circuit in which the newborn resides. Having neurons that undergo long term potentiation more easily could lead to these circuits firing more due to their increased sensitivity (strengths of synapses). The accompanying result is a downstream behavioral, sensory, or cognitive change that results from the acquisition or strengthening of a circuit that did not formerly exist or was not formerly strong.
As of now research is being conducted into the methods that dictate when neurogenesis should occur, and what clinical consequences improperly regulated neurogenesis may have. There are many questions left to be answered, leaving the puzzle far from complete. However, a clearer picture is starting to emerge.