The spinal cord is like a highway, sending and receiving information between the brain and the whole body. Our abilities to sense, move, and react are all transmitted as information along this highway. This neural connection is what allows for the travel of signals from the central nervous system, the main highway, to the peripheral nervous system, or the many exits that branch out to different regions of the body. The signals enable control of bodily functions, from big movements like taking a stride, to the fine motor control needed to play the piano skillfully. Impactful traffic jams on the highway can occur if a disruption, like a dilapidated road, stops the traffic from moving. As is with injury to the spinal cord, a disruption to the flow of neural traffic results in varying degrees of damage.
Spinal cord injuries (SCIs) occur in a variety of ways. Significant trauma, whether it is an athletic mishap, falling from significant heights, physical violence, gun violence, or motor vehicle accidents can cause SCI [1]. Most injuries to the spinal cord in earlier eras have been the result of trauma, war, and strenuous labor. The consequences of injury include reduced sensation, paralysis, and a diminished autonomy to carry out tasks [1]. Mapping out the multiple routes and avenues that pave the spinal cord and its extension throughout the body, along with the location of the injury on the spinal cord, is necessary to understand the effects of SCI on the body.
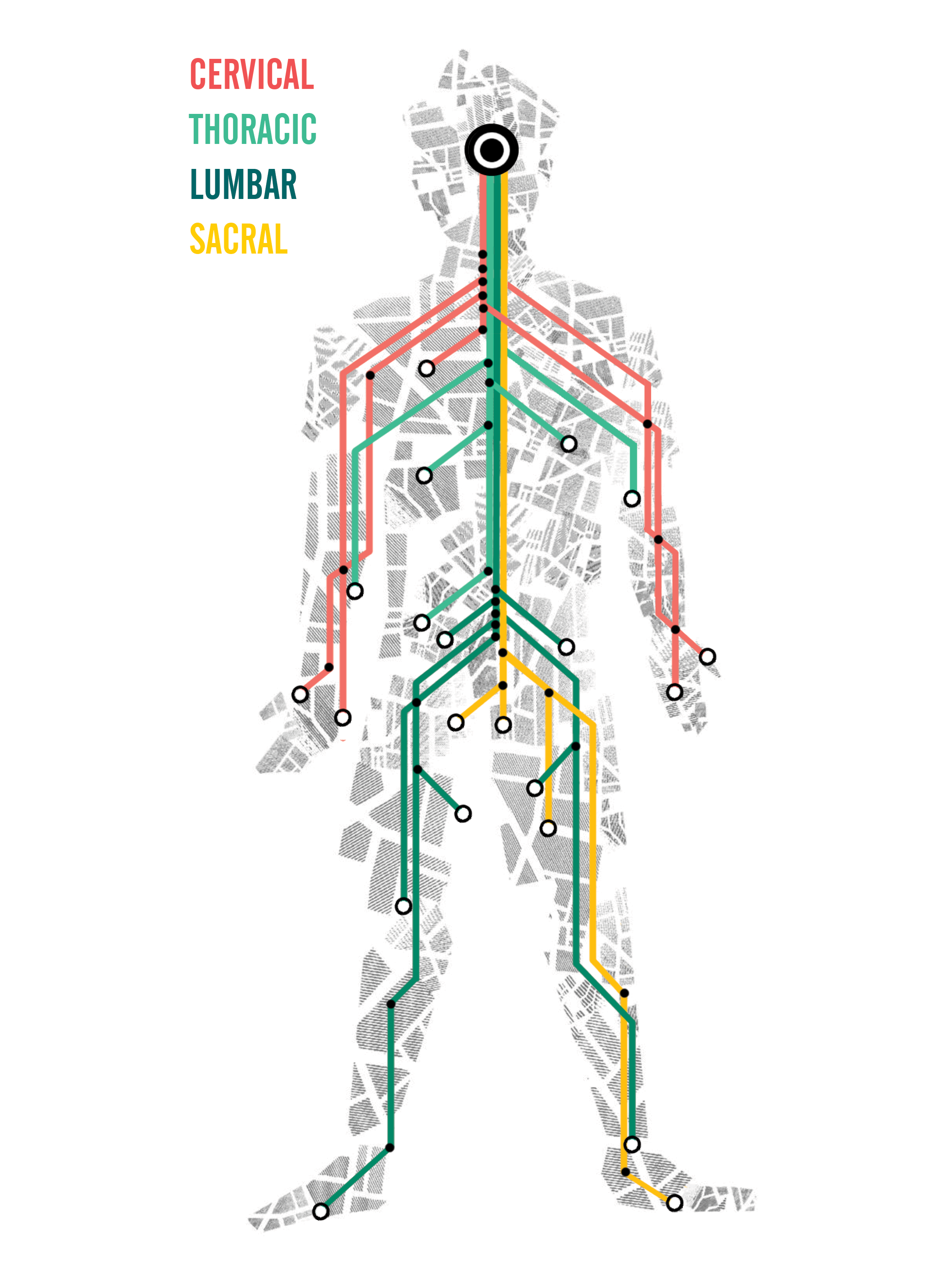
Avenues of the Vertebrae
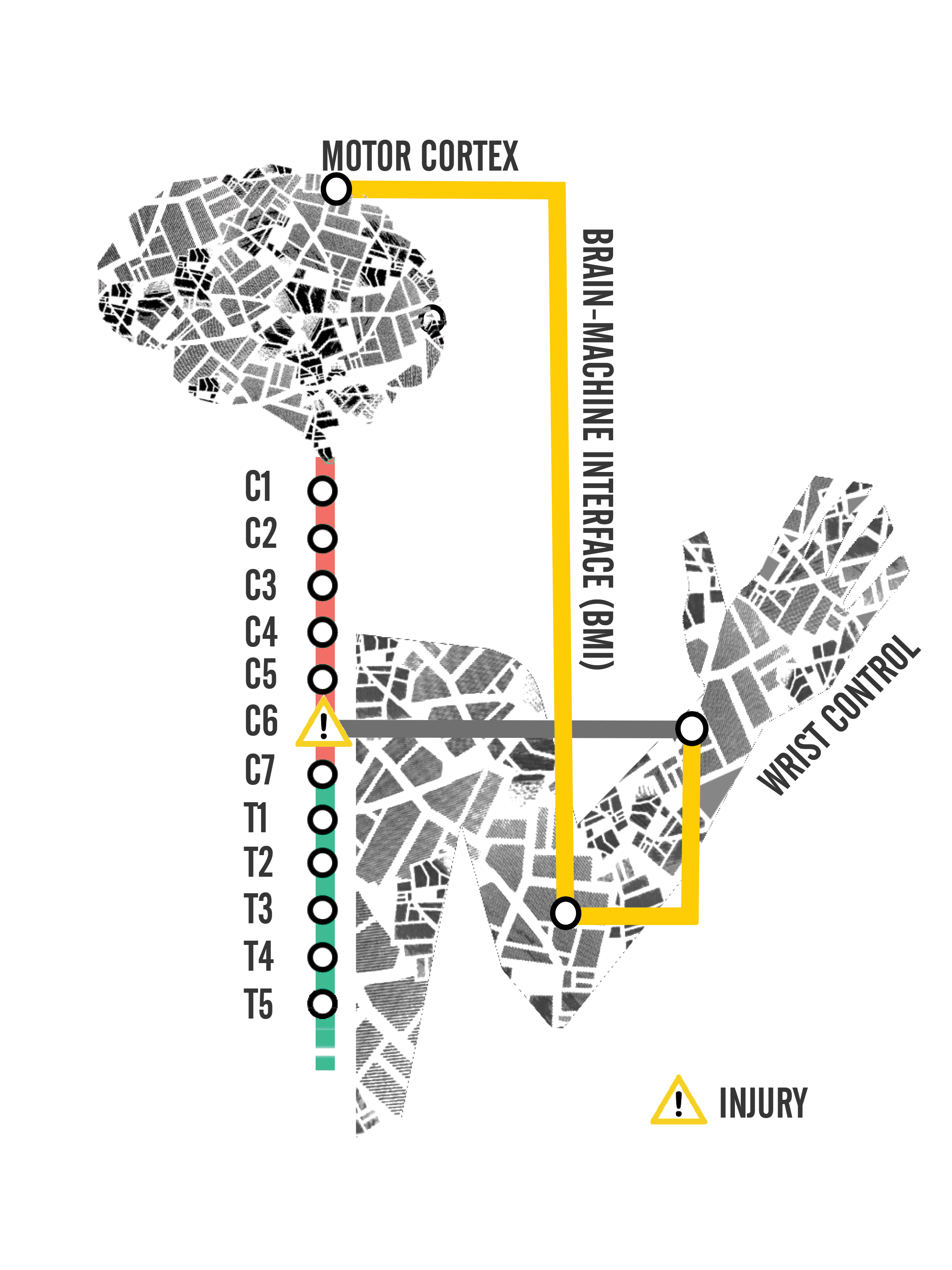
From the neck down, there are four sections of the spinal cord highway: the cervical, the thoracic, the lumbar, and the sacral. The vertebrae, the bones surrounding the spinal cord, split the spinal cord into smaller sections, and each region is associated with different motor and sensory functions. Hippocrates, the Grecian Father of Medicine, noted in his literature Anatomy of the Spine that there is an articulation of nerves and vessels that make up the spinal cord, providing information as to how our wiring reflects the functionality of the human body and how an injury could have a negative impact [1]. The location of the SCI affects the degree of severity to a person’s motor function, as well as which body parts are affected. The higher the injury is on the spinal column, the greater the disruption to neural activity, which in turn affects the function of the entire body.
Starting from the bottom portion of the vertebrae is the sacral region. Injuries in this region generally result in some loss of function in the hips and legs, but the ability to walk is more than likely maintained [2]. Control of bladder and bowel functions may be disrupted due to damage in this region, causing incontinence. However, incontinence can be treated using equipment such as catheters to help alleviate urinary stress [1].
Next up are the lumbar vertebrae, which serve as the trunk of the spinal cord. Injuries to the lumbar nerves generally result in reduced function in the hips and legs, as well as in bowel movement and bladder control. If leg muscle control and strength do not support the body in standing or walking, a wheelchair may be necessary for mobility.
Further up are the thoracic vertebrae, which trail up most of the back. The nerves from this region are associated with the upper chest, mid-back, the hands, and the abdominal muscles. Injuries to the thoracic spine will disrupt control of movement in those areas. Since disruption will also affect neural connectivity below, control of legs, bowels, and the bladder are also significantly reduced or lost. Paralysis from the waist down due to a thoracic SCI is what causes paraplegia. People with damage to the thoracic column still retain upper forelimb function, or most control of the arms, and would most likely use a manual wheelchair to navigate independently. Other accommodations that are available include modified cars, standing frames, crutches, and braces.
The final region that stands at the top of the human spine are the cervical vertebrae, which support the neck. Injury at the cervical level causes the most severe damage, disabling movement from the neck down. Internal functions such as breathing, speaking, and bladder control may be reduced as well. Paralysis of the legs and arms lead to tetraplegia, commonly known as quadriplegia. Living with injury at this level often requires use of a powered wheelchair, along with complete assistance from a caretaker for activities of daily living.
As observed, injuries high on the spinal cord have a severe effect on the body and its ability to function. Living with reduced neural traffic flow in the spinal cord impacts a person’s mobility and ability to accomplish daily tasks. People affected with SCI make various adjustments to their lifestyle depending on where their injury affects them.
By the mid-20th century, medical advancements in sterilization, surgery, and technology forwarded innovations for treatment in the field of spinal cord medicine. The breakthroughs made in trauma care and treatments came with the promise of promoting survivability and improving quality of life.
Current Treatments
Historically, the effects of SCI have proved to be incredibly difficult to reverse. Treatments were limited and failed to help people with SCI survive its debilitating effects. Egyptians used bronze catheters for urinary drainage, which was the earliest form of treatment. Various forms of eastern medicine have been documented to alleviate spinal inflammation through use of splints and bedrest [1]. Catheters and wheelchairs have endured as devices that help those with SCI maintain an accessible and manageable quality of life. However, numerous medical and technological advancements helped make current treatments accessible for people with SCI today. Surgical advancements in the early 20th century have made the preventative means against spinal cord injuries possible. This includes spinal cord fusion surgeries, which are performed to brace the spinal cord using compression rods to treat scoliosis, spinal fractures, and dislocations. However, surgeries alone had little effect in promoting function in SCI patients [1].
More recently, functional electric stimulation (FES) has been explored and used in clinical studies to promote muscle movements. FES involves electric stimulation of muscular contractions via superficial placement of electrodes. Electrodes are taped onto the skin, at the site of interest (like an arm or leg) to help stimulate the muscles for supportive movement and exercise [3]. Stimulation at the lumbar cord can assist in generating responses and step like movements at the legs [4]. FES has been used in therapies for the lower limbs to facilitate standing, walking, or cycling, whereas upper limb systems can enable functional grasping as well as limited, close-range arm movements [3].
Physical therapy, equipment, community, and self-care continue to be large components to the management of daily life for people with SCI. New discoveries and innovations in spinal cord rehabilitation bring us closer to uncovering solutions that could possibly remedy injury. Not only do treatments and continuous study help people with SCI manage life, but they also open a new road, or in the least a detour around what was damaged, to restore independence and improve quality of life.
Developing a Detour
Current research has focused on methods of stimulating the spinal cord to promote the restoration of limb function lost to injury. Just as was done with FES to stimulate muscle movements, techniques involve looking at the effects of electrostimulation and how it promotes the repair of neurons’ connectivity to each other [5].
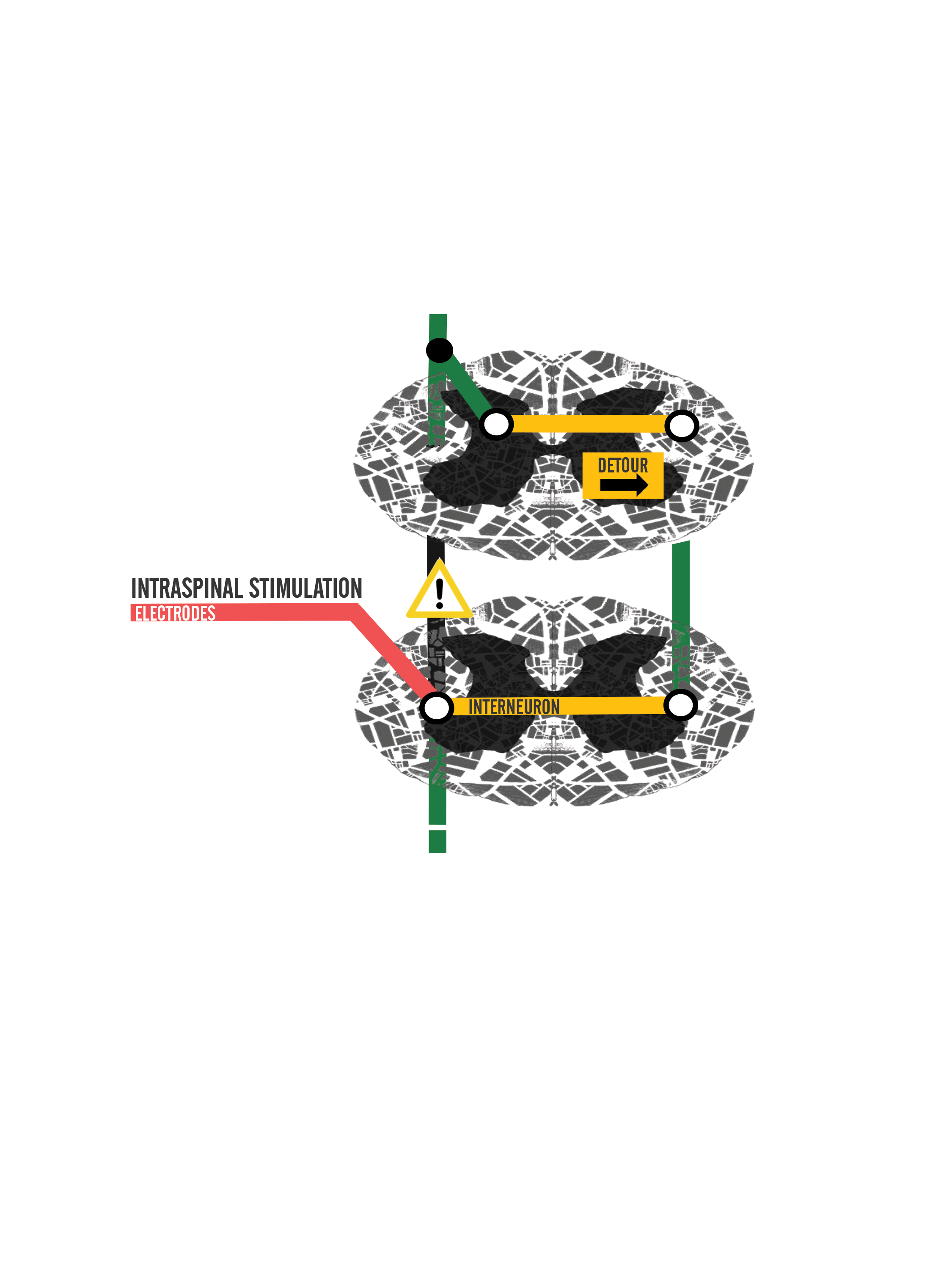
Neuro-prosthetics can be implemented to promote neural activity via electrostimulation. This kind of electrostimulation involves application of subcutaneous (into the skin) or transcutaneous (onto the skin) electrodes to an area of interest to receive electric impulses [3]. Forms of neuro-prosthetics include brain-machine interface (BMI) and neuro-modulatory stimulation [2]. When there’s a disruption in the road, traffic congests and mobility is halted. But if a detour is created around the road block, traffic flows again. These two forms of neuro-prosthetics can act to replace a blocked highway and allow for new traffic to flow and promote movement of the muscles.
A BMI is a device that records and decodes signals from the brain, which enables control of assistive devices, like a robotic arm. Signals from the brain towards the desired output veers away from the site of injury, allowing for neural communication to flow towards the effector muscle [2]. One experiment that focused on the capability of BMIs was conducted on monkeys [5]. A neural block was injected into a monkey to temporarily paralyze its wrist muscles. The monkey played a computer video game that required it to grasp and move a mouse to place the arrow in a box on the screen. The application of a brain controlled spinal interface made it possible to send signals from brain cell activity directly to motor neurons innervating the muscle cells. The regions of brain cell activity were tracked based on the monkey’s wrist movement [5]. This was done by guiding the traffic of signals from the electrodes at the brain to the electrodes at the effector muscles. Data showed that movement was still possible with the BMI helping the monkey successfully use its wrist to complete the computer task [5]. Using a BMI is slower than taking the natural spinal cord highway, but nonetheless, the use of a BMI detour provides an effective measure of relieving spinal traffic congestion.
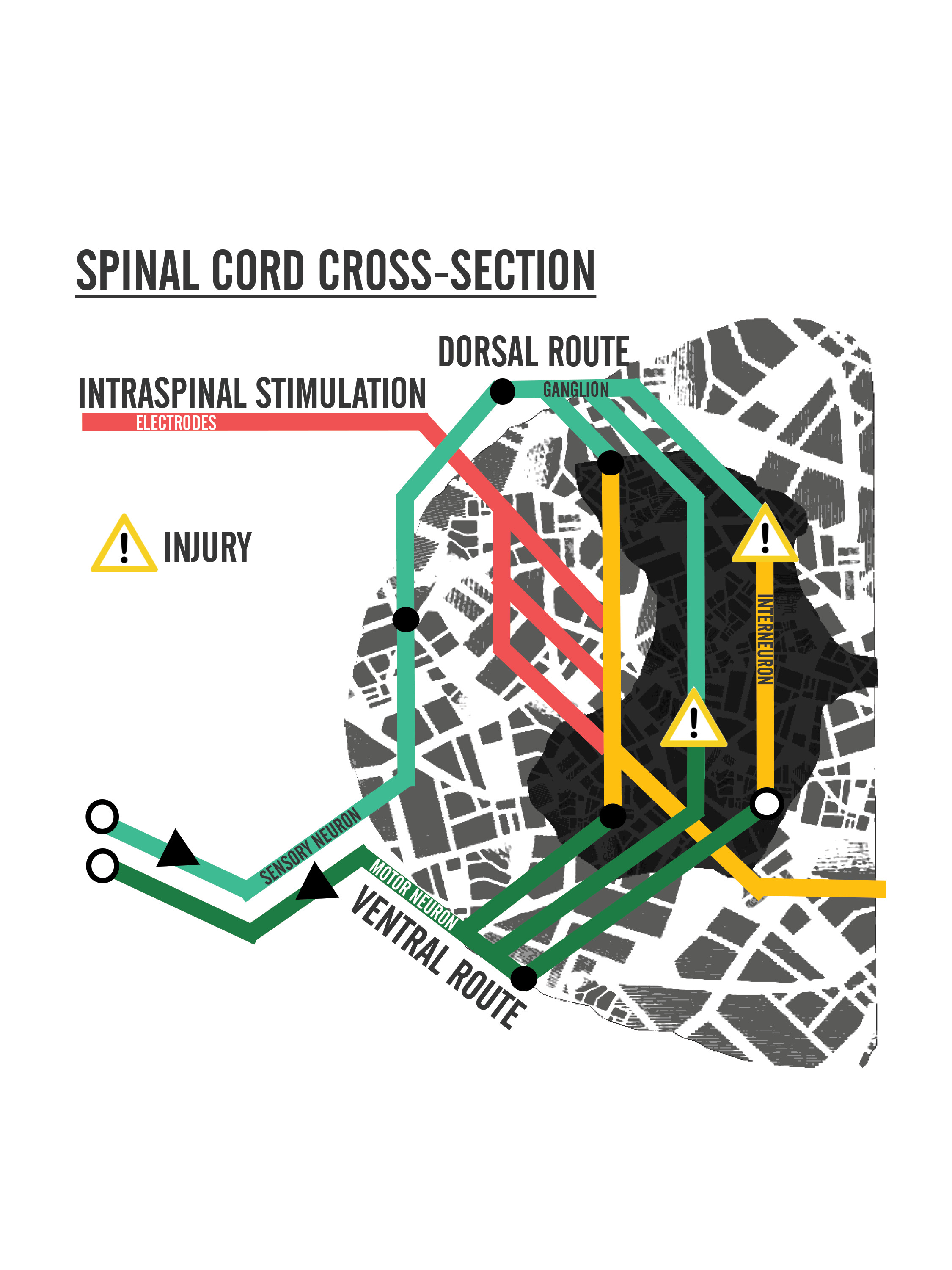
The neuro-modulatory system is another neuro-prosthetic which relies more on the modification of the spinal cord highway itself to promote neural connectivity [2]. Two major forms of neuro-modulatory stimulation include epidural and intraspinal microstimulation. Epidural refers to the outer layer of the spinal cord, whereas intraspinal means within the spinal cord. Both forms assist in promoting functional limb movements through the restructuring of spinal circuitry. Epidural stimulation is where electrodes are placed on a vertebra. Intraspinal stimulation is more invasive than epidural stimulation, requiring electrode implantation to the spinal cord grey matter [2,3]. Studies performed on rats investigate axonal regrowth and regeneration triggered by electric stimulation during rehabilitation following injury [2,6]. In one study, the injection of enzymes like Chondroitinase demonstrated possible promotion of neural plasticity and improved muscle coordination following injury [7]. Additionally, Chondroitinase may possibly play a role in modifying glial cells, which help neurons form synaptic connections with surrounding neurons and build more intersections to allow for multi-route travel [8]. It is with high hopes that these conducted treatments may improve neural plasticity, or the ability for neurons to regenerate their own tissue and to modify their synaptic strengths, following trauma [3].
A combination of BMI and neuro-modulatory stimulation at the spinal cord provides a more natural promotion of motor neurons to replace the damaged ones [9]. But because injury at different regions along the spinal cord exhibit varying degrees of severity, the same treatment will almost always have differing levels of efficiency between individual injuries. Clinical studies are considering spinal stimulation using transcutaneous electrodes for human patients to reduce harm and cost of treatment [4]. As more research moves towards clinical trial, more study is necessary to determine what efficiency of movement can be maintained using neuro-prosthetics [10].
Current research is driving forward to investigate strategies in improving quality of life for paralyzed patients with SCI. Neuro-prosthetic techniques show the potential to form detours that serve as newly constructed routes and paths in the spinal cord for stimulating muscle movement and control. Hopefully, continued research into SCI will help produce a remedy for the effects of injury and restore function where it has been lost.
Conclusion
Although spinal cord injuries are far from being completely curable, developing treatments have paved new paths - mapping out the possibilities of restoring bodily functions for many people with spinal cord injuries. Fine motor control, large movements, and tactile senses all have the potential to be restored. All the while, education on living with SCI, including managing equipment, finding clinical treatments, community involvement, and the practice of self-care, continues to be necessary for people with SCI to help maintain quality of life. Study of the spinal cord has come a long way in the last century. Researchers continue to find new ways to repair and modify damaged tissue after injury and allow for communication between the brain and the body through a combination of neuro-prosthetics, enzymatic interventions, and electric stimulation promoting neural connectivity [3,6,8,4]. It will only be a matter of time until the road, once damaged by trauma and disease, is repaired and neural traffic on the highway can resume moving on the right track.
References
- Donovan, W. (2007). Spinal Cord Injury—Past, Present, and Future. Journal of Spinal Cord Medicine, 85-100.
- Jackson, A. & Zimmermann, J. B. Nat. Rev. Neural interfaces for the brain and spinal cord—restoring motor function. Neurol. 8, 690–699 (2012); published online 13 November 2012
- Brus-Ramer, M., Carmel, J., Chakrabarty, S., Martin, J. (2007). Electrical Stimulation of Spared Corticospinal Axons Augments Connections with Ipsilateral Spinal Motor Circuits after Injury. The Journal of Neuroscience, 13793-13801.
- Gerasimenko, Yury, Ruslan Gorodnichev, Tatiana Moshonkina, Dimitry Sayenko, Parag Gad, and V. Reggie Edgerton. “Transcutaneous electrical spinal-cord stimulation in humans.” Annals of Physical and Rehabilitation Medicine (2015).
- Moritz, C., Lucas, T., Perlmutter, S., Fetz, E. (2006). Forelimb Movements and Muscle Responses Evoked by Microstimulation of Cervical Spinal Cord in Sedated Monkeys. J Neurophysiol, 110-120. Retrieved January 8, 2017.
- Gad, Parag, Jaehoon Choe, Mandheerej Singh Nandra, Hui Zhong, Roland R. Roy, Yu-Chong Tai, and V. Reggie Edgerton. “Development of a multi-electrode array for spinal cord epidural stimulation to facilitate stepping and standing after a complete spinal cord injury in adult rats.” Journal of neuroengineering and rehabilitation 10, no. 1 (2013): 2.
- Jefferson, Stephanie, Nicole Tester, and Dena Howland. "Chondroitinase ABC Promotes Recovery of Adaptive Limb Movements and Enhances Axonal Growth Caudal to a Spinal Hemisection." The Journal of Neuroscience (2011): 5710-720. Print.
- Bradbury EJ, Carter LM (2011) Manipulating the glial scar: chondroitinase ABC as a therapy for spinal cord injury. Brain Res Bull 84:306–316.
- Mushahwar VK, Horch KW. Selective activation and graded recruitment of functional muscle groups through spinal cord stimulation. Ann NY Acad Sci 860: 531–535, 1998.
- Prochazka A, Mushahwar VK, Yakovenko S. Activation and coordination of spinal motoneuron pools after spinal cord injury. In: Neural Repair and Recovery, edited by McKerracher L, Douchet G, Rossignol S. Amsterdam: Elsevier, 2002, p. 109–124.