The Role of Mirror Neurons in Empathy, Social Learning, and Autism Spectrum Disorder
Have you ever flinched when watching a character get hurt on TV? Yawned when someone near you yawned? These reactions may be due to unique brain cells known as mirror neurons [1] [2]. These cells activate both when you perform a specific action yourself or observe someone else perform that same action. In this way, mirror neurons do what their name suggests: mirror the perceived actions of others. When you yawn in response to your sleep-deprived friend or flinch at a scary episode of Criminal Minds, your mirror neuron system (MNS) is activated, responding and projecting its responses to motor regions that ultimately lead to your behavioral reaction. When these mirror neurons malfunction, social learning, communication, and sensory processing may suffer, changing the way individuals perceive and interact with others and their environment. These behaviors might manifest in a variety of disorders, one such example being Autism Spectrum Disorder (ASD). Reflecting on the purpose, function, and responses of these unique mirror neurons may offer a treatment target for ASD and other sensory processing diseases or disorders.
The Discovery of Mirror Neurons
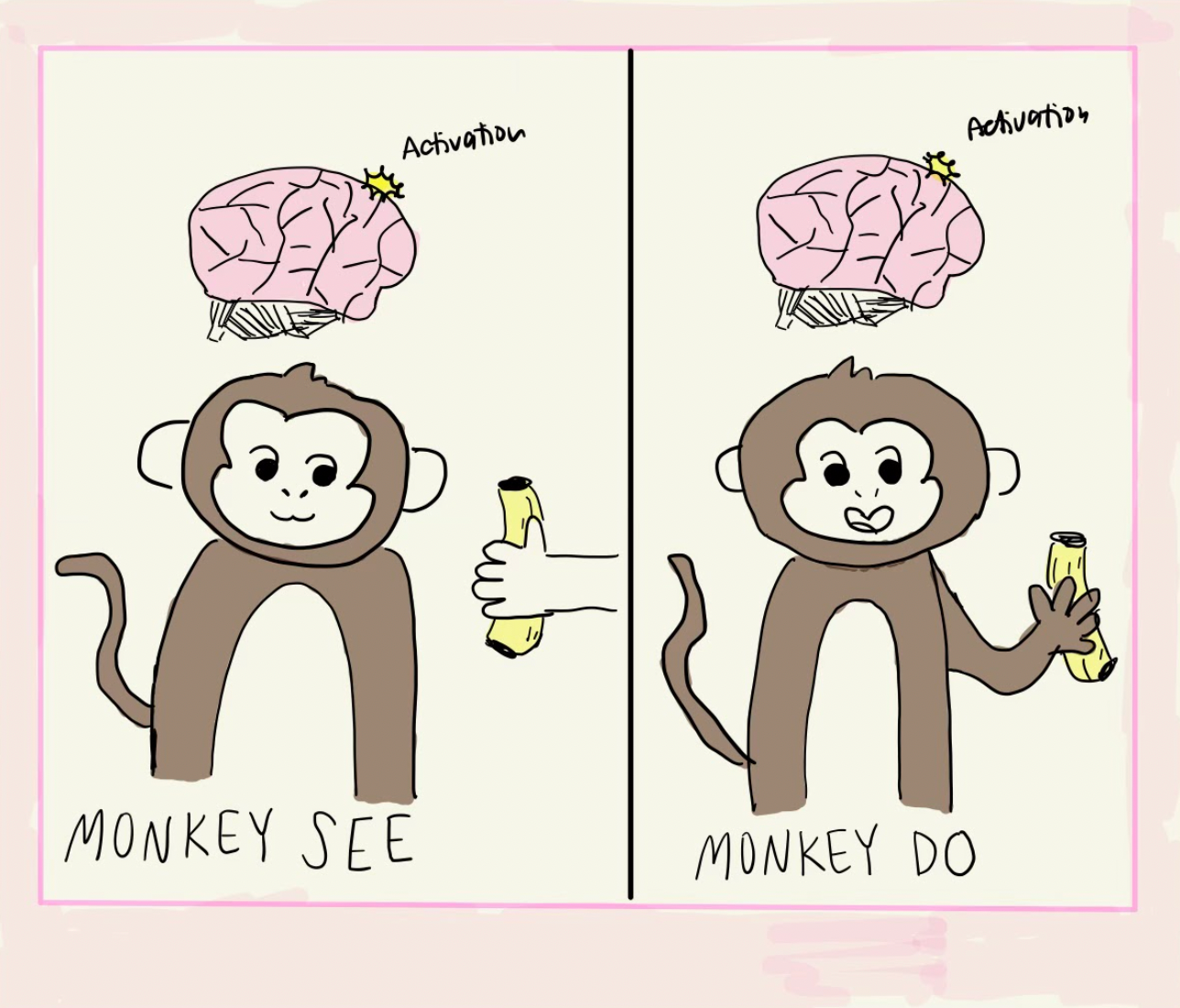
In the early 1990s, mirror neurons were first discovered in monkeys tasked with grasping and moving an object [2]. While the monkeys performed and observed this task, the researchers used non-invasive functional magnetic resonance imaging (fMRI) to measure the monkey’s neural activity. fMRI functions by measuring changes in blood flow. High neuronal firing uses more energy, which requires oxygen. This oxygen is replenished by the transportation of oxygenated blood to the brain region in use. fMRI measures these changes in blood flow, leading brain regions in high use to appear “lit up” on fMRI imaging [3]. When the monkeys performed the grasping task, motor regions F5 and F6 of their brain lit up on fMRI. The region known as F5 controls the monkey’s hand movements, while F6 is responsible for the preparation of reaching and grasping movements [4] [5]. Interestingly, the same regions were also activated when the monkey watched the experimenter’s hand perform the grasping task, as if the monkey had performed the action themselves. Electrophysiology experiments that recorded the firing activity of individual neurons in regions F5 and F6 indicated that 92 out of the 532 total recorded neurons fired both when the monkey performed the task and when they watched the experimenter perform the action with their hand. The researchers called these neurons “mirror neurons,” as they mirror the actions of others as if the observer. Interestingly, these neurons had little to no activity when the monkey observed the researcher grasping the object using a tool other than their hands. Hence, the mirror neuron response was specific to the exact interaction between the researcher’s hand and the target object of action, indicating that mirror neuron activity is highly specialized for empathic or imitative situations [2].
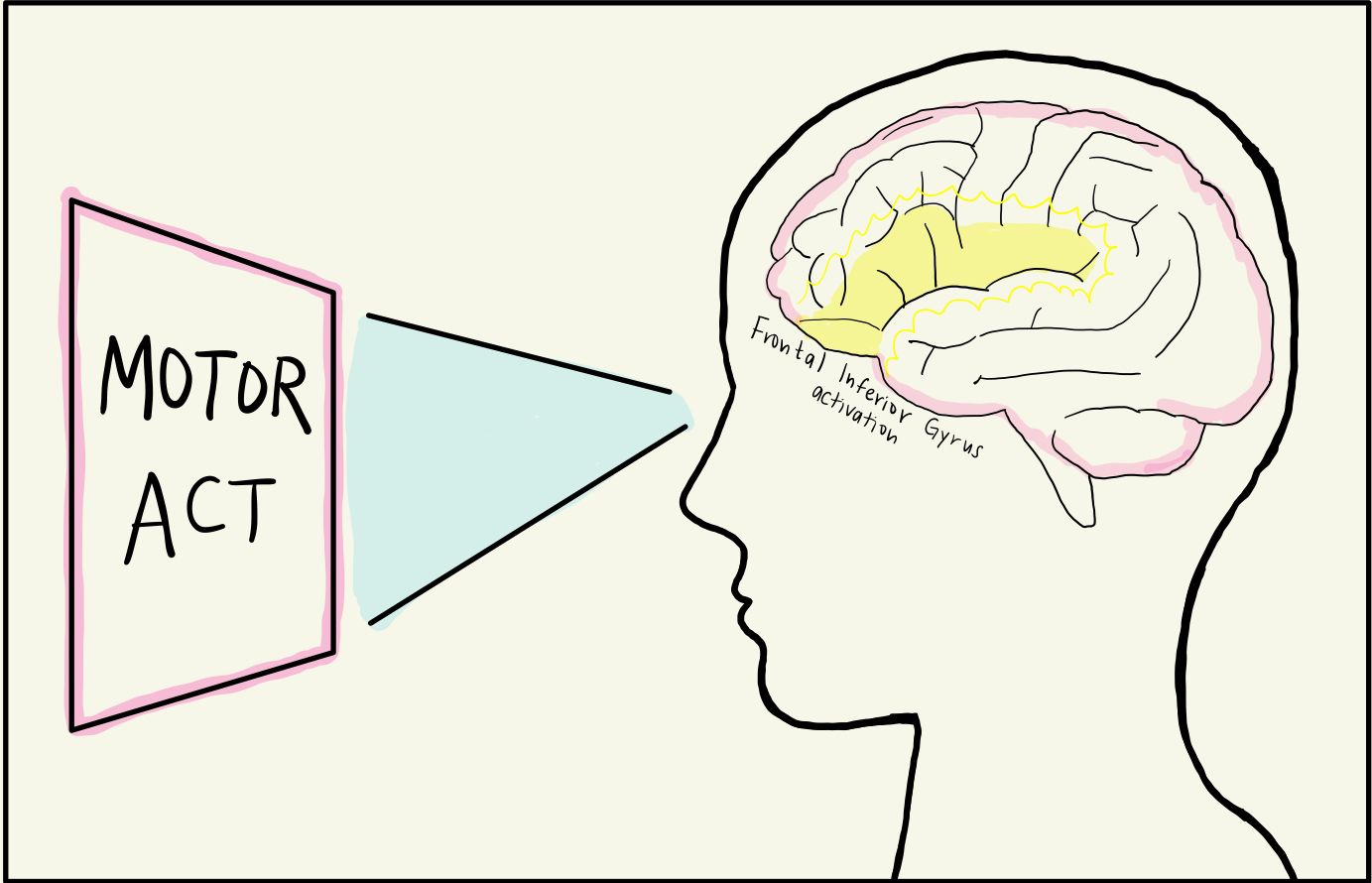
Neuroscientists then wondered if these mirror neurons fired in response to other stimuli, such as observing scenes just before an action takes place. One group hypothesized that mirror neurons primed people for action, and tested this by displaying various images of a person picking up a cup to human subjects [6]. The first image depicted a person lifting a spilling cup from a messy table, suggesting that the person in the image was cleaning. The second image depicted a person lifting the cup on a clean, set table where the cup was full, indicating that the person in the image was about to take a sip of their drink. The experimenters wanted to know if these different intentions surrounding the same action changed the mirror neural activity of the observer. In both scenarios, fMRI depicted that the mirror neurons in the observer’s inferior frontal gyrus were activated, preparing the observer to pick up the cup in anticipation. This indicated that mirror neurons are not only present in the brain’s motor cortex, but also in other regions such as the frontal lobe. The inferior frontal gyrus, found in the frontal lobe, is dense with mirror neurons and responsible for attention, motor imagery, speech and language, and social cognitive functions [7]. While the MNS was activated in both the spilling and drinking context, the activation was stronger for the context of drinking from the cup, likely due to drinking being more evolutionarily relevant to survival than cleaning [7][8].
The discovery of mirror neurons emphasized how our brains are constantly trying to anticipate and understand the intentions of others by firing in response to the context of an action, allowing us to predict others’ actions and needs [6]. Humans are innately social and need to cooperate for survival. This ability of the MNS to predict and understand the behavior of others allows us to quickly imitate and learn new tasks, empathize with others, and react accordingly in social settings. The inferior frontal gyrus and several other brain regions have mechanisms in place whose job is to mirror the actions and intentions of others [7]. Understanding the mechanisms behind mirror neurons that help guide social behaviors is pivotal to treating conditions with social deficits that may be due to MNS dysfunction. Researchers have found this system malfunction to have a range of varying symptoms that affect one's social learning and communication abilities, as well as induce abnormal sensory-motor behaviors.
Mechanisms Within the Human Mirror Neuron System
The inferior frontal gyrus is a key player in the MNS [6]. With it, there is also the inferior parietal lobule that integrates sensory information into motor output, the amygdala that processes emotions, and any higher-order visual and auditory processing that is present in the occipital and temporal lobes [9]. Ultimately, these brain regions turn sensory feedback into a mirrored neural response.
When watching your sleep-deprived friend yawn in class, the neurons in your inferior frontal gyrus become activated due to visual feedback from your occipital lobe. More specifically, the Brodmann area 9 (BA9) within your inferior frontal gyrus is activated, converting visual information into a mental image of the action [10][11]. Another possible path is the activation of the mirror neurons in the inferior frontal gyrus via hearing a yawn, meaning the BA9 receives auditory input from the temporal lobe, turning the sound of a nearby yawn into a mental response. Humans, some primates, and other animals such as mice or rats have the unique ability to mentalize, that is, think about and represent the actions, emotions, or mental state of ourselves and others. This is known as the “theory of mind,” allowing humans to approximate what those around them are thinking and feeling [12]. In this way, when your friend yawns, mirror neurons in your inferior frontal gyrus respond to input from the senses and project this input to motor regions, leading you to yawn involuntarily. This is a mirrored, imitative behavior due to a neural mirrored response. This automatic imitation is known as a form of echophenomena. The exact neural mechanism behind echophenomena is unknown [13].
Ultimately, it is hypothesized that mirror neurons are activated due to sensory inputs that lead to the mentalization of an action, behavior, or emotion. This mentalization allows us to understand the perspectives of others, helping us to form our social responses.
Mirror Neurons in Action
As previously mentioned, yawning is contagious due to the MNS and it is speculated that this response is due to auditory and visual input to the inferior frontal gyrus. Contagious yawning appears to be more prevalent among familiar faces such as friends and family members; the more empathy you grant someone, the more likely you are to yawn contagiously. This mirroring behavior is also gender-biased, with an individual responding more to others of the same gender than to a different gender [14]. Similar to the monkey’s MNS responding to the researcher’s hand and not the tool, the MNS responds to what it is familiar with. The more familiar the task or the individual, the more likely and intensely an observer’s MNS will respond.
Similar to yawning, laughing is highly contagious due to visual and auditory input to the brain [15]. While you can laugh spontaneously in response to a funny TikTok or TV show, we are much more likely to laugh when in groups than on our own. Preschool children have been shown to laugh eight times as much and smile three times as much in the company of their peers compared to on their own [15]. Seeing others laugh and smile activates a brain region known as the pregenual anterior cingulate cortex, one of the brain regions responsible for processing emotions [16]. This region uniquely interprets laughter from others and uses the MNS to elicit more laughter from the individual. This emotional mirroring system in the cingulate cortex allows the observer to directly perceive what those around them are feeling, defining a biological basis for empathy. This emotion mirroring for happiness is similar to the mechanisms for mirroring disgust, fear, and anger, and is referred to as facial mimicry [17]. Simply put, this concept demonstrates how seeing someone smile leads you to imitate their facial expression and also smile. It is theorized that this mimicry may be due to mechanisms within the MNS that project to motor regions, stimulating facial muscles to mimic the facial expression being observed [17]. Facial expressions are a key component of communicating and understanding emotions nonverbally. If this MNS process is functioning differently in autistic individuals, it may explain why they tend to report struggling with non-verbal communication and emotion recognition [18]. It is important to note that ASD is a spectrum, emphasizing that the extent to which this MNS process may be altered will vary from person to person, leading to different presentations of the same altered neural pathway.
While the MNS is activated by perceiving the actions and emotions of others, it can also be modulated by our own biases. For example, we tend to empathize less with those we do not like [19]. In a study where the researcher betrayed the human subject in an economic game, the subject rated the researcher as unfair, unpleasant, unlikable, and unattractive [19]. Interestingly, when this unsportsmanlike researcher was subjected to an electrical pain stimulus delivered to their right hand, the male subjects observing the researcher in pain had significant, visible fMRI brain activity in the nucleus accumbens, an area dense in mirror neurons that is important for processing reward [19]. Essentially, the male subjects felt rewarded seeing the person who betrayed them in pain. The researchers called this the “revenge response,” however, it was not significantly prevalent in the female subjects. Both male and female subjects displayed less fMRI neural activity related to pain empathy when the unsportsmanlike researcher was in pain compared to when a sportsmanlike researcher was in pain, further emphasizing how this system is modulated by our own feelings and biases [19].
Recent studies in mice have demonstrated a similar concept with aggression [20]. Mirror neurons present in the male mouse ventromedial hypothalamus respond to aggression performed by the mouse itself and others. Essentially, the ventromedial hypothalamus, a small brain region within the hypothalamus that is dense in hormone receptors and drives aggression and social fear behaviors, is active when the male mouse is fighting and when it observes fighting between other individuals [21] [22]. To test this, fiber photometry was used to record the neural activity of the mouse when it was fighting and when it observed two other mice fighting through a transparent divider [20]. Fiber photometry allows for in vivo recordings of calcium levels, which correspond to neural activity since calcium is released during a synapse [20]. The researchers went one step further and activated the mirror neurons in the male mouse ventromedial hypothalamus, leading the male to display increased territorial aggression towards male intruders in addition to increased aggression towards atypical individuals such as female mice and the male mouse’s own mirror image. This was especially surprising since male mice would usually want to display mating behaviors toward female mice instead of attacking them. When these neurons were silenced, the male mouse demonstrated diminished aggression and attacked a male intruder about a third of the time. Interestingly, these same neurons did not trigger aggression in female mice when activated [20].
Modulating the MNS seems to alter an individual’s state, social behavior, and empathy. When the MNS malfunctions, these social, emotional, and behavioral functions of the MNS may also malfunction and manifest in a variety of symptoms, as may be the experience of autistic individuals.
Autism Spectrum Disorder
ASD may develop due to an early deficit in the mirror neuron system [1]. As some researchers have found, this system malfunction has a range of varying symptoms that affect one’s social learning and communication abilities and induce abnormal sensory-motor behaviors [1]. These symptoms lie along a spectrum that can manifest as a variety of behaviors, such as hyperfixation behaviors or an inability to understand the implied meaning of metaphors [23]. Many scientists have hypothesized that mirror neurons play a significant role in other symptoms related to ASD, as they allow for the unconscious learning of others’ social behaviors [1]. They allow us to relate personal experiences with the actions we observe in others. We take what we see others do and understand it through the lens of our past encounters. People with ASD may not benefit from the same unconscious learning as neurotypical people because of their challenges when it comes to understanding others’ actions [24].
Autistic individuals experience innate dysfunction of the MNS, which can lead to difficulties with imitation, empathy, and language. Notably, the ability to imitate others and exhibit empathy is disrupted in autistic individuals. As mentioned, autistic individuals may struggle to differentiate facial expressions and may not experience common social behaviors, such as contagious yawning [1] [24].
In one study, scientists were interested in how individuals with ASD reacted to visual stimuli, such as a video of a person tapping their fingers, and auditory stimuli at different frequencies [25]. In the study, they tested individuals diagnosed with ASD and controls without such diagnoses. The participants were instructed to lift their fingers in response to the varying stimuli. In both tasks, autistic individuals reacted slower than the control group. The researchers hypothesized that this is due to either a lack of automatic imitation or the imitation of meaningless actions. This includes imitating a different kind of behavior than those without ASD. For example, some autistic individuals will repeat words or noises they hear multiple times in what is called echolalia. Researchers found that visual and auditory sensory processing is impaired in those with ASD. This could relate to issues in processing specific stimuli, which leads to either hyperimitation or timing difficulties. The study also found that the issue is related to imitative control abilities as opposed to an overall imitative skill deficit, exemplifying how autistic individuals have trouble regulating the level of imitation they exhibit, rather than an issue with imitation as a whole [25].
Research into the involvement of mirror neurons in ASD can help determine symptoms that may not be as obvious during an autism diagnosis. Autism is overwhelmingly underdiagnosed in women, despite having a similar prevalence of autism in both sexes [26]. For every four males, only one woman gets diagnosed. Researchers hypothesize that this is due to females’ ability for camouflaging autism symptoms. This camouflage ability is how well an individual can mask symptoms by observing others’ behavior and copying them. Because the diagnostic criteria are sufficiently biased toward men, a lot of women go undiagnosed well into their adult years. Autistic men are more likely to show a lack of empathy and the desire and intent to form friendships with others. On the contrary, autistic women are more likely to have a higher level of social motivation and mimic facial expressions. This is possibly due to social expectations and reinforcements that are more influential for women than men. Another reason could be genetic differences that improve women’s ability to camouflage traditional autistic behavior [27]. For example, some autistic women perform an overwhelming amount of eye contact rather than a decreased level because of societal pressure. In an attempt to fit into norms, some autistic women may overcompensate. Future mirror neuron and ASD research may help uncover the root cause of underdiagnosed women with ASD.
Conclusion
Whether it be contagious yawning or mirrored social behaviors, the MNS is constantly working to anticipate, understand, and perceive the actions and emotions of others, especially those we are most familiar with.
Social learning is impacted when the MNS experiences deficits. ASD research is currently focused on developing early interventions to improve social learning in autistic children. One example is the Early Start Denver Model (ESDM) [27]. This intervention emphasizes joint play and routines between adults and autistic children. They complete the same activities in a mirroring way, involving everything from vocal and body movement imitation to sharing and turn-taking of an activity. This therapy engages the sensory and motor systems in the mirror-neuron-dense areas of the developing brain [27]. Thus, the child experiences increased social learning to strengthen sociability.
Another understudied intervention utilizing the function of mirror neurons is Action Observation Treatment (AOT) [28]. This treatment does what its name implies: patients in rehabilitation from neurological issues watch a video of a person doing an action and then replicate it. This utilizes the MNS and helps form pathways in the brain that can lead to an increase in motor function [28]. This can help people post-stroke or those with Parkinson’s who are rehabilitating movement. Although currently understudied, AOT is a prime example of how studying the MNS can lead to better intervention for more people, not just those with Autism.
While scientists are still reflecting on the role of mirror neurons in ASD and social learning, the MNS is a promising target for not only ASD intervention, but potentially other diseases and disorders where individuals experience social, emotional, or motor deficits such as stroke, ALS, Parkinson’s, Schizophrenia, phantom limb pain, etc. The MNS also serves as a neurobiological basis for empathy, a pillar of the human experience. Mirror neurons remind us that empathy is not solely based on shared experiences, but is rooted in evolutionarily special brain cells whose role is to mentally mirror another individual’s actions or state to promote social bonds, understanding, and compassion.
References
- Perkins, T., Stokes, M., McGillivray, J., & Bittar, R. (2010). Mirror neuron dysfunction in autism spectrum disorders. Journal of clinical neuroscience: official journal of the Neurosurgical Society of Australasia, 17(10), 1239–1243. doi.org/10.1016/j.jocn.2010.01.026
- Gallese, V., Fadiga, L., Fogassi, L., & Rizzolatti, G. (1996). Action recognition in the premotor cortex. Brain: a journal of neurology, 119 ( Pt 2), 593–609. https://doi.org/10.1093/brain/119.2.593
- Amanamba U,, Sojka A,, Harris S,, Bucknam, M. and Hegdé J. (2020). A Window Into Your Brain: How fMRI Helps Us Understand What Is Going on Inside Our Heads. Frontiers for Young Minds, 8, 484-603. doi.org/10.3389/frym.2020.484603
- Rizzolatti, G., & Fadiga, L. (1998). Grasping objects and grasping action meanings: the dual role of monkey rostroventral premotor cortex (area F5). Novartis Foundation symposium, 218, 81–103. doi.org/10.1002/9780470515563.ch6
- Albertini, D., Gerbella, M., Lanzilotto, M., Livi, A., Maranesi, M., Ferroni, C. G., & Bonini, L. (2020). Connectional gradients underlie functional transitions in monkey pre-supplementary motor area. Progress in neurobiology, 184, 101-699. doi.org/10.1016/j.pneurobio.2019.101699
- Iacoboni, M., Molnar-Szakacs, I., Gallese, V., Buccino, G., Mazziotta, J. C., & Rizzolatti, G. (2005). Grasping the intentions of others with one's own mirror neuron system. PLoS biology, 3(3), e79. doi.org/10.1371/journal.pbio.0030079
- Hartwigsen, G., Neef, N. E., Camilleri, J. A., Margulies, D. S., & Eickhoff, S. B. (2019). Functional Segregation of the Right Inferior Frontal Gyrus: Evidence From Coactivation-Based Parcellation. Cerebral cortex (New York, N.Y. : 1991), 29(4), 1532–1546. doi.org/10.1093/cercor/bhy049
- Proverbio, A. M., & Zani, A. (2022). Mirror Neurons in Action: ERPs and Neuroimaging Evidence. In P. S. Boggio (Eds.) et. al., Social and Affective Neuroscience of Everyday Human Interaction: From Theory to Methodology. (pp. 65–84). Springer. doi.org/10.1007/978-3-031-08651-9_5
- Rajmohan, V., & Mohandas, E. (2007). Mirror neuron system. Indian journal of psychiatry, 49(1), 66–69. doi.org/10.4103/0019-5545.31522
- Monticelli, M., Zeppa, P., Mammi, M., Penner, F., Melcarne, A., Zenga, F., & Garbossa, D. (2021). Where We Mentalize: Main Cortical Areas Involved in Mentalization. Frontiers in neurology, 12, 712532. doi.org/10.3389/fneur.2021.712532
- Haker, H., Kawohl, W., Herwig, U., & Rössler, W. (2013). Mirror neuron activity during contagious yawning--an fMRI study. Brain imaging and behavior, 7(1), 28–34. doi.org/10.1007/s11682-012-9189-9
- American Psychological Association. (2023). APA Dictionary of Psychology. American Psychological Association. https://dictionary.apa.org/theory-of-mind
- Brown, B. J., Kim, S., Saunders, H., Bachmann, C., Thompson, J., Ropar, D., Jackson, S. R., & Jackson, G. M. (2017). A Neural Basis for Contagious Yawning. Current biology: CB, 27(17), 2713–2717.e2. doi.org/10.1016/j.cub.2017.07.062
- Norscia, I., Zanoli, A., Gamba, M., & Palagi, E. (2020). Auditory Contagious Yawning Is Highest Between Friends and Family Members: Support to the Emotional Bias Hypothesis. Frontiers in psychology, 11, 442. doi.org/10.3389/fpsyg.2020.00442
- Addyman, C., Fogelquist, C., Levakova, L., & Rees, S. (2018). Social Facilitation of Laughter and Smiles in Preschool Children. Frontiers in psychology, 9, 1048. doi.org/10.3389/fpsyg.2018.01048
- Caruana, F., Avanzini, P., Pelliccia, V., Mariani, V., Zauli, F., Sartori, I., Del Vecchio, M., Lo Russo, G., & Rizzolatti, G. (2020). Mirroring other's laughter. Cingulate, opercular and temporal contributions to laughter expression and observation. Cortex; a journal devoted to the study of the nervous system and behavior, 128, 35–48. doi.org/10.1016/j.cortex.2020.02.023
- Rymarczyk, K., Żurawski, Ł., Jankowiak-Siuda, K., & Szatkowska, I. (2019). Empathy in Facial Mimicry of Fear and Disgust: Simultaneous EMG-fMRI Recordings During Observation of Static and Dynamic Facial Expressions. Frontiers in psychology, 10, 701. doi.org/10.3389/fpsyg.2019.00701
- Drimalla, H., Baskow, I., Behnia, B., Roepke, S., & Dziobek, I. (2021). Imitation and recognition of facial emotions in autism: a computer vision approach. Molecular autism, 12(1), 27. doi.org/10.1186/s13229-021-00430-0
- Singer, T., Seymour, B., O'Doherty, J. P., Stephan, K. E., Dolan, R. J., & Frith, C. D. (2006). Empathic neural responses are modulated by the perceived fairness of others. Nature, 439(7075), 466–469. doi.org/10.1038/nature04271
- Yang, T., Bayless, D. W., Wei, Y., Landayan, D., Marcelo, I. M., Wang, Y., DeNardo, L. A., Luo, L., Druckmann, S., & Shah, N. M. (2023). Hypothalamic neurons that mirror aggression. Cell, 186(6), 1195–1211.e19. doi.org/10.1016/j.cell.2023.01.022
- Hashikawa, Y., Hashikawa, K., Falkner, A. L., & Lin, D. (2017). Ventromedial Hypothalamus and the Generation of Aggression. Frontiers in systems neuroscience, 11, 94. doi.org/10.3389/fnsys.2017.00094
- Silva, B. A., Mattucci, C., Krzywkowski, P., Cuozzo, R., Carbonari, L., & Gross, C. T. (2016). The ventromedial hypothalamus mediates predator fear memory. The European journal of neuroscience, 43(11), 1431–1439. doi.org/10.1111/ejn.13239
- Lord, C., Elsabbagh, M., Baird, G., & Veenstra-Vanderweele, J. (2018). Autism spectrum disorder. Lancet (London, England), 392(10146), 508–520. doi.org/10.1016/S0140-6736(18)31129-2
- Helt, M. S., Sorensen, T. M., Scheub, R. J., Nakhle, M. B., & Luddy, A. C. (2021). Patterns of Contagious Yawning and Itching Differ Amongst Adults With Autistic Traits vs. Psychopathic Traits. Frontiers in psychology, 12, 645310. doi.org/10.3389/fpsyg.2021.645310
- Schunke, O., Schöttle, D., Vettorazzi, E., Brandt, V., Kahl, U., Bäumer, T., Ganos, C., David, N., Peiker, I., Engel, A. K., Brass, M., & Münchau, A. (2016). Mirror me: Imitative responses in adults with autism. Autism, 20(2), 134–144. doi.org/10.1177/1362361315571757
- Hull, L., Petrides, K.V. & Mandy, W. The Female Autism Phenotype and Camouflaging: a Narrative Review. Review Journal of Autism and Developmental Disorders, 7, 306–317 (2020). doi.org/10.1007/s40489-020-00197-9
- Vivanti, G., & Rogers, S. J. (2014). Autism and the mirror neuron system: insights from learning and teaching. Philosophical transactions of the Royal Society of London. Series B, Biological sciences, 369(1644), 20130184. doi.org/10.1098/rstb.2013.0184
- Bonini, L., Rotunno, C., Arcuri, E., & Gallese, V. (2022). Mirror neurons 30 years later: implications and applications. Trends in cognitive sciences, 26(9), 767–781. doi.org/10.1016/j.tics.2022.06.003