Introduction
Imagine a world where depression's relentless grip is broken, where hope can finally shine through the clouds of despair. That was, unfortunately, not something Ms. T could afford to imagine. Ms. T was a 27-year-old woman who was struck with misfortune when a drunk driver drove through a red light at high speeds and crashed into her [1].
Upon collision, Ms. T lost consciousness for several hours, after which, she woke up in the hospital where she would spend the next week recovering from the accident. While Ms. T had no history of prior mental health problems, the doctors determined she had suffered a traumatic brain injury (TBI). She was able to make a speedy recovery, however, and was discharged from the hospital a week later. Six months following the injury, Ms. T unexpectedly began to develop an increasingly depressed mood, difficulty sleeping, and loss of interest, which interfered with her day-to-day life [1]. Eventually, she sought out the help of a psychiatrist. After receiving a routine psychiatric assessment, she was diagnosed with moderate-to-severe major depression. Because selective serotonin reuptake inhibitors and other antidepressants are a common treatment for depression following TBI, Ms. T was prescribed several antidepressant medications over fourteen years with hopes of improving her condition. However, these medications were ultimately ineffective in alleviating her depressive symptoms [1].
As Ms. T’s improvement began to plateau, she was informed of an alternative treatment for her major depressive episodes [1]. Namely, she was eligible to participate in an ongoing repeated transcranial magnetic stimulation (TMS) clinical trial. The study recruited participants to investigate the therapeutic efficacy and safety of a new active TMS approach stimulating both sides of the affected brain region in patients with treatment-resistant depression. Now 41 years old and running out of options, one can imagine Ms. T was eager to be free of her despondent affliction and eagerly agreed to participate in the trial [1].
Noninvasive form of brain stimulation, operating completely outside of the body. The procedure uses magnetic fields to stimulate nerve cells in specific areas of the brain.
Transcranial Magnetic Stimulation Through Time
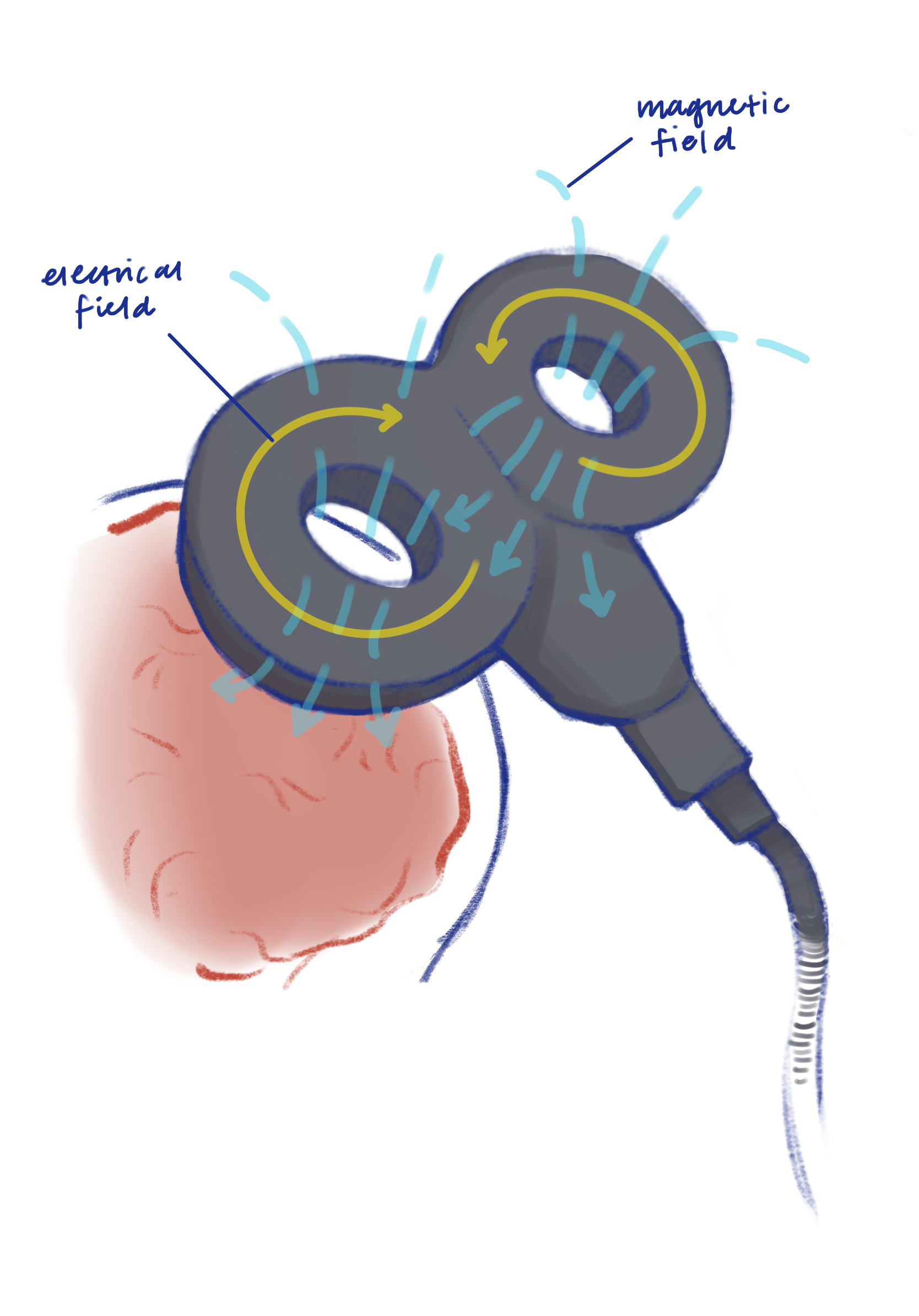
The origin of Ms. T’s treatment can be traced back to 1985 when researchers began using magnetic fields to stimulate the brain using a technique called TMS [2]. Researchers passed an electric current through a circular coil placed flat on the scalp to produce a precise magnetic pulse that would cross the skull and electrically stimulate the outermost layers of the brain, called the cortex, without needing to insert electrodes into the brain [2].
In 1990, researchers improved TMS even further by devising a more precise and focused way to distribute TMS-induced electrical currents in the brain. By rapidly alternating magnetic fields in a coil, researchers found they could induce a more targeted electrical current in the brain to stimulate the outermost brain layers [3]. In the following years, TMS was slowly but surely refined through the optimization of the shape of TMS coils and the use of high-frequency magnetic pulses. These advancements improved the safety, accuracy, and control of brain stimulation [3].
Mechanisms of Action
Before Ms. T could undergo TMS treatment, however, Magnetic Resonance Imaging (MRI) of her brain was first performed to understand the structure and function of her brain and better guide her TMS treatment [1]. Interestingly, the MRI indicated the tearing of long connecting nerve fibers called axons in the white matter connective tissues. Such tearing is common in cases of traumatic brain injury and is associated with the disruption of connections between cortical areas such as the prefrontal cortex, a region of neurons affecting personality and behaviors, and the hippocampus, a region of neurons known for memory storage, to other regions of the brain. In Ms. T’s case, researchers believed that changes in white matter connections underlined the development of depression. Namely, the prefrontal cortex was an area of interest as it is the frontmost area of the brain that regulates thoughts, actions, and emotions. However, stimulating the back and the sides of Ms. T’s prefrontal cortex using magnetic fields not only made the underactive brain region more active but also enhanced Ms. T’s disrupted connectivity and excitability of the white matter in the surface and deeper brain regions [1].
While the history of TMS provides a foundation for the treatment, understanding the mechanism of the TMS procedure helps provide insight into how the treatment can be used to treat depression. At its fundamental level, TMS uses electric currents in a coil to produce magnetic fields that induce a subsequent electric current in the outer layer of the brain [5]. Although the prefrontal cortex is the target of TMS treatment, clinicians must first calibrate TMS by stimulating the motor cortex, a brain region that is responsible for regulating movement, which produces an observable muscle jerk and informs clinicians the TMS is working [5]. Furthermore, the motor cortex is thoroughly mapped and easily identifiable, making it an ideal candidate for calibration [5].
To stimulate the motor cortex, TMS produces magnetic waves that bypass the skull and induce an electric current in the brain, first activating the interneurons in the outermost layers of the brain that then send signals to activate pyramidal neurons [6]. Pyramidal neurons are tear-drop-shaped brain cells responsible for receiving and processing information from other neurons and transmitting it to the rest of the nervous system, supporting cognitive processes like perception, attention, learning, and memory. Synchronous activation of many pyramidal neurons results in electrical impulses passing from neurons in the motor cortex to the spine through the peripheral nerves, eventually reaching the target muscle and triggering a movement. To ensure the TMS device is placed over the correct target region of the brain and fine-tuned to the optimal strength, clinicians gradually increase the magnetic field stimulating the motor cortex until a finger jerk or another muscle contraction is produced [6]. What is fascinating is that from the activated interneuron to the target muscle, the whole process takes only about 10 milliseconds, which is remarkably fast compared to an average human blink lasting 100-150 milliseconds.
Now with an established anchor point at the motor cortex, the clinician can move on to target the back and sides of the prefrontal cortex. TMS generates a magnetic field of alternating strength that then passes through the skull and to the back and side regions of the prefrontal cortex located just behind the forehead [6]. The bent or curved shape of axons of neurons in the prefrontal cortex allows them to function as a coil and allows TMS to induce a highly targeted and precise electric current that can vary with rapidly alternating magnetic fields. These high-frequency electromagnetic pulses cause prefrontal inhibitory interneurons to be less active and pyramidal neurons of the prefrontal cortex that help control cognitive and emotional behavior to be more active [6]. Understanding how TMS uses magnetic pulses to precisely stimulate underactive neurons in regions like the motor and prefrontal cortex helps us understand how TMS can lead to therapeutic effects, particularly in the treatment of depression.
Therapeutic Effects
Depression, as in Ms. T’s case, is often associated with volume reduction and the atrophy of the hippocampus and prefrontal cortex, regions involved in voluntary motor movements and the integration of sensory information [7]. For instance, prolonged stress during depression often involves the release of stress hormones, including cortisol, which can concentrate in the hippocampus. High cortisol levels in animal studies are linked to toxicity and damage of neurons, especially those of the hippocampus, causing them to shrink and die [8]. Chronic stress has also been linked to decreased neuron formation and decreased number of synapses and dendrites [9].
By exciting pyramidal neurons while deactivating inhibitory interneurons, TMS can increase the activity of the underactive prefrontal cortex. Activation and excitation of the prefrontal cortex enables TMS to enhance the plasticity and connectivity of affected neurons, leading to a decrease in symptoms of depression [10]. Furthermore, TMS can enhance the density and growth of axons, dendrites, and synapses. These enhanced neurons may stimulate and induce gene expression and enzyme production, promoting the production of neurotransmitters, receptors, and neuromodulators that are low during depression and are involved in mood regulation, pleasure, and reward [10]. By elevating the levels of neurotransmitters such as dopamine, glutamate, norepinephrine, and serotonin in the brain, TMS helps improve mood, motivation, and reward processing. Even further, TMS has been shown to decrease elevated cortisol levels associated with prolonged stress and anxiety in depression [10]. Combined, the multifaceted effect of TMS allows it to enhance the growth, health, connectivity, and plasticity of neurons and induce a subsequent increase in brain volume that was lost during the depression.
Neuroplasticity
As we dive deeper into the mechanisms and pathways underlying the therapeutic effects of TMS, we should not ignore an intriguing link between these intricate processes and the ability of TMS to enhance the brain's ability to change the activity and connectivity of networks involved in depression through growth and reorganization.
TMS owes its non-invasive nature to the capacity of the magnetic field to induce short-lasting electrical impulses across the skull and throughout the targeted outermost brain regions [12]. These short electrical pulses disrupt surrounding neural activity while inducing neurons to fire electrical impulses in a specific direction or pattern. As time passes, the neurons firing these impulses grow stronger through various processes such as myelination or remyelination, where the neurons develop more insulation for the axon to improve the speed and strength of electric signals. At the same time, the inactivated neurons are slowly broken down or reorganized, weakening pathways commonly active in neurological disorders like depression. Such a restructuring of the neural network is a form of neuroplasticity, a cornerstone to TMS’s capabilities in reducing the symptoms of various neurological disorders by habituated brain patterns and activities [12].
Additionally, TMS's effect on reorganizing the neural network depends on variations of Brain-Derived Neurotrophic Factor (BDNF) expression across different neurons [12]. BDNF is a molecule that is essential for the growth, migration, and differentiation of neurons and plays a key role in neuron reorganization [12]. The molecule plays a part in inducing neurons to change shape or reconnect with other neurons to form new neuron connections [10]. For instance, a 2023 study conducted by the Weizmann Institute of Science assessed the correlation between the frequency of TMS on BDNF expression to changes in plasticity and connectivity of the hippocampus, a portion of the frontal lobe that aids in decision-making known as the prelimbic cortex, and a central brain structure that aids in balance and movement known as the striatum [13]. Researchers found that the frequency of TMS is associated with induced neuroplasticity, as an increasing TMS frequency corresponded to an increased expression and concentration of BDNF [13]. Surprisingly, the study also found that anesthetized animals undergoing TMS expressed lower levels of BDNF than animals who were awake for the procedure [13]. Therefore, it is possible that patients such as Ms. T, who are mainly awake throughout the procedure, allow TMS-induced electrical impulses to more clearly define and disrupt targeted brain activities [14].
How Effective is Transcranial Magnetic Stimulation?
Throughout four weeks, Ms. T received a total of 20 low-frequency repetitive TMS procedures [1]. By the end of the 4th week of treatment, Ms. T had more than a 40-50% reduction in her depressive symptoms [1]. Moreover, Ms. T also experienced improvements in mood, speed of information processing, and verbal fluency with no adverse effects [1]. These were undeniable improvements in her depression and her social, personal, and professional life. With the help of the same treatment Ms. T received, many patients suffering from treatment-resistant depression are now turning to TMS to significantly reduce their depression and improve their quality of life [1].
The success of TMS in treating depression can be further emphasized using a recent Stanford University study that examined the safety and efficacy of intermittent theta-burst stimulation (iTBS) in tackling treatment-resistant depression. iTBS works by delivering a series of brief and intermittent, but high-frequency magnetic pulses to the brain [15]. Researchers found that using iTBS helped 86% of patients experience a noticeable improvement in depression symptoms and 79% of patients experienced a full remission with no severe adverse effects [15]. Not only did TMS improve symptoms of depression, but it also noticeably improved the verbal processing speed that is often impaired in depression [15].
Another interesting case to consider is a large 2022 US study that examined the effects and safety of TMS in US military veterans suffering from both depression and PTSD [16]. After administering TMS to 770 of these veterans, 41.4% had a significant decrease in depression symptoms, and 20% experienced full remission. Likewise, 65.3% of 521 veterans experienced a significant reduction in PTSD symptoms and 46.1% achieved PTSD remission. With good tolerability and no adverse effects, the study supports that TMS may be a safe and effective treatment for both depression and PTSD in large sample sizes [16].
Interestingly, several studies have also examined the use of TMS for post-stroke depression in chronic stroke survivors. For instance, a 2021 Australian study examined the possibility of using high-intensity rTMS of the parietal cortex in post-stroke depression [17]. The parietal cortex, vital for sensory perception and integration, was targeted since a stroke here often leads to depression and cognitive impairment. Following 10 sessions of rTMS, the treatment group experienced a significant reduction in depression and improved cognitive function compared to no significant reduction in the placebo group. Researchers observed that brain connectivity and neuron communication in the right parietal cortex were also improved [17]. Combined, the results of the study highlight the multifaceted effect of TMS and its promising ability to ameliorate depression.
While it is important to recognize studies highlighting the efficacy of TMS, it is equally important to address its limitations in different age groups. For instance, a 2021 US study examined how effective TMS is on the left prefrontal cortex as a therapy for treatment-resistant depression in 103 adolescents [18]. While TMS treatment was relatively harmless, it was only slightly more effective in treating depression than the placebo. Namely, in both the active TMS and placebo groups, around 40% of patients experienced an improvement in symptoms and around 29% experienced full remission [18]. Thus, while TMS produced clinically significant improvement in depression symptoms, it failed to show similar results in adolescent depression treatment and remission effectiveness, suggesting further research is required for TMS use in adolescents and children [18].
Additional Applications
While it is true that TMS is primarily used to treat treatment-resistant depression, recent research also indicates that TMS is a promising treatment for a myriad of additional conditions, ranging from Parkinson’s and Alzheimer’s diseases to stroke and heart arrhythmia. Consider even the case of Ms. T, who became the first recorded case of a traumatic brain injury (TBI) patient successfully treated for depression with TMS [1]. Successfully expanding the use of TMS to treat post-TBI depression in Ms. T helped reduce the previous fears that TMS may be too dangerous to use in TMS patients due to the elevated risk of seizure induction [19]. Just as Ms. T’s case helped pave the way for applying TMS to more neurological conditions, researchers are actively transforming the application of TMS in a spectrum of neurological disorders, targeting not only the exterior brain tissue responsible for depression but also deeper brain structures.
Similar to Ms. T’s intractable major depressive disorder, obsessive-compulsive disorder (OCD) is another condition that remains a challenge to treat. Currently, 30 - 60% of OCD patients do not respond to psychotherapy or medication, prompting researchers to turn to deep transcranial magnetic stimulation (dTMS) for solutions [20]. In one 2021 multicenter Israeli-American study, researchers found that dTMS improved OCD symptoms in over 70% of OCD patients initially and in over 50% of OCD patients one month after the treatment [21]. At the same time, researchers looked into using rTMS for adjuvant priming, wherein an initial brief stimulation would prepare the brain to be more responsive to subsequent prolonged stimulation and improve the efficacy of TMS [22]. The researchers found that using adjuvant priming rTMS over the supplementary motor area resulted not only in a significant decrease in OCD symptoms but also a decrease in symptoms of depression and anxiety [22].
Alzheimer’s disease is one of the most studied and prevalent neurological conditions, affecting 27 million Americans while having limited effective treatment or cure. However, researchers are now studying TMS as a potential candidate for Alzheimer’s treatment. One 2021 Italian study explored the use of H-Coil in rTMS treatment for Alzheimer’s Disease [23]. An H-coil is an experimental type of magnetic coil designed to target deep brain structures that are involved in memory and cognition [23]. By using such a coil, researchers found that TMS over frontal-parietal-temporal lobes could improve cognition and memory in Alzheimer’s patients for up to two months [23]. A later study conducted in China looked at whether precise rTMS over the Left Parietal Cortex would be a better target for improving memory and cognitive function in Alzheimer’s disease [7]. Researchers found that TMS applied over the parietal cortex significantly improved cognition, memory recall, and time orientation in Alzheimer’s patients. Overall, high-frequency TMS was found to elicit faster results, greater symptom improvement, and greater tolerability.
TMS is also emerging as a prominent treatment for neuropathy. Marked by weakness, pain, and numbness, neuropathy is a debilitating condition that damages nerves outside of the brain and spinal cord. Several studies, such as a 2021 French study, examined how safe and effective TMS is in treating neuropathy when applied to the primary motor cortex and dorsolateral prefrontal cortex [24]. Interestingly, researchers found that TMS applied to the primary motor cortex but not the dorsolateral prefrontal cortex was able to significantly improve pain relief, sensory dimension of pain, self-reported pain intensity, and fatigue. While safe and well-tolerated, both TMS approaches did not affect the type of pain, mood, sleep, or quality of life.
A promising treatment target of TMS is also executive function deficits in autism. To examine how safe and effective TMS is in improving higher-level cognitive skill deficits, including deficits in problem-solving, attention control, and flexible thinking associated with autism, a 2021 Canadian study treated youth and young adults with autism using rTMS [25]. While the researchers did not find a clear improvement in executive function following TMS, they did find that patients who had more difficulty with higher-level cognitive skills in everyday tasks at the beginning of the study appeared to benefit more from the rTMS treatment.
Cognitive impairment in the early stages of Parkinson’s Disease is also an emerging treatment target for TMS. One 2019 Canadian study, for example, was interested if iTBS used for depression treatment can also be used to improve cognitive deficits in Parkinson’s Disease when applied to the dorsolateral prefrontal cortex [24]. While previous studies showed that TMS leads to short-term improvement in cognitive performance for specific tasks, this study found that iTBS produced significant improvements in overall cognitive performance, namely improving attention and visual perception of the spatial relationships of objects in Parkinson’s patients. Remarkably, these improvements lasted for up to one month following the procedure [24].
Interestingly, there is also a potential to treat disorders of consciousness, such as comas, vegetative states, and minimally conscious states, using TMS. To see if TMS could improve impaired consciousness, a 2021 Chinese study applied rTMS to the left dorsolateral prefrontal cortex of patients with disorders of consciousness [25]. Unfortunately, rTMS was effective in improving consciousness in only some but not all treated patients, and ultimately rTMS did not improve the awakening ratio for patients with impaired consciousness [25].
Although one would think that magnetic stimulation would only treat neurological conditions, results from a 2016 Chinese study suggest that noninvasive magnetic stimulation may also be effective in improving cardiac conditions [26]. In the study, researchers applied magnetic stimulation to the left stellate ganglion (LSG) of dog subjects to examine the therapeutic effect on abnormal heart rhythm following a heart attack. LSG is a small cluster of nerve cells located in the neck and regulates the heart's rhythm and rate, as well as blood pressure and breathing. The researchers found that magnetic stimulation of LSG successfully reduced the incidence of irregular heartbeat in the ventricle of the canine heart by inhibiting the neuronal activity of the hyperactive LSG. This study indicates that magnetic stimulation is a potential noninvasive alternative for the existing implantable cardiac devices that treat cardiovascular conditions, like heart arrhythmia.
Conclusion
Providing an impetus for developing a safe, effective, and lasting treatment for depression, TMS may give relief where pharmacotherapy fails. It is probable that Ms.T had never imagined that she would suffer many episodes of major depressive disorder over 14 years following her TBI, but perhaps even less likely she anticipated having her brain repeatedly pulsed with a magnetic field. It may be even more difficult for one to amass any hope or prospect of joy or resolution when in a state of depression. Painlessly surpassing the skull and stimulating the affected brain region, TMS works to alter the activity, connectivity, communication, and excitability of affected neurons in depression. In addition to being painless and well-tolerated, many patients have reaped unprecedented improvements in their symptoms of depression, allowing them to return to their normal professional, social, emotional, and physical activities and faculties. The ability of TMS to stimulate and modulate the activity and connectivity of superficial brain regions has also garnered interest in applying the procedure for other neurological disorders, such as PTSD and Alzheimer’s, that sometimes have limited treatment success. Thus, with each magnetic pulse and each excited neuron, TMS is helping pave the way for a painless, effective, and long-lasting treatment for depression and many other neurological disorders.
References
- Fitzgerald, P. B., Hoy, K. E., Maller, J. J., Herring, S., Segrave, R., McQueen, S., Peachey, A., Hollander, Y., Anderson, J. F., & Daskalakis, Z. J. (2011). Transcranial magnetic stimulation for depression after a traumatic brain injury: a case study. The journal of ECT, 27(1), 38–40. doi.org/10.1097/YCT.0b013e3181eb30c6
- Barker, A. T., Jalinous, R., & Freeston, I. L. (1985). Non-invasive magnetic stimulation of human motor cortex. Lancet (London, England), 1(8437), 1106–1107. doi.org/10.1016/s0140-6736(85)92413-4
- Tofts P. S. (1990). The distribution of induced currents in magnetic stimulation of the nervous system. Physics in medicine and biology, 35(8), 1119–1128. doi.org/10.1088/0031-9155/35/8/008
- Blumberger, D. M., Vila-Rodriguez, F., Thorpe, K. E., Feffer, K., Noda, Y., Giacobbe, P., Knyahnytska, Y., Kennedy, S. H., Lam, R. W., Daskalakis, Z. J., & Downar, J. (2018). Effectiveness of theta burst versus high-frequency repetitive transcranial magnetic stimulation in patients with depression (THREE-D): a randomised non-inferiority trial. Lancet (London, England), 391(10131), 1683–1692. doi.org/10.1016/S0140-6736(18)30295-2
- Chail, A., Saini, R. K., Bhat, P. S., Srivastava, K., & Chauhan, V. (2018). Transcranial magnetic stimulation: A review of its evolution and current applications. Industrial psychiatry journal, 27(2), 172–180. doi.org/10.4103/ipj.ipj_88_18
- Klomjai, W., Katz, R., & Lackmy-Vallée, A. (2015). Basic principles of transcranial magnetic stimulation (TMS) and repetitive TMS (rTMS). Annals of physical and rehabilitation medicine, 58(4), 208–213. doi.org/10.1016/j.rehab.2015.05.005
- Zhang, F. F., Peng, W., Sweeney, J. A., Jia, Z. Y., & Gong, Q. Y. (2018). Brain structure alterations in depression: Psychoradiological evidence. CNS neuroscience & therapeutics, 24(11), 994–1003. doi.org/10.1111/cns.12835
- Xu, B., Lang, L. M., Li, S. Z., Guo, J. R., Wang, J. F., Wang, D., Zhang, L. P., Yang, H. M., & Lian, S. (2019). Cortisol Excess-Mediated Mitochondrial Damage Induced Hippocampal Neuronal Apoptosis in Mice Following Cold Exposure. Cells, 8(6), 612. doi.org/10.3390/cells8060612
- Eisch, A. J., & Petrik, D. (2012). Depression and hippocampal neurogenesis: a road to remission?. Science (New York, N.Y.), 338(6103), 72–75. doi.org/10.1126/science.1222941
- Chervyakov, A. V., Chernyavsky, A. Y., Sinitsyn, D. O., & Piradov, M. A. (2015). Possible Mechanisms Underlying the Therapeutic Effects of Transcranial Magnetic Stimulation. Frontiers in human neuroscience, 9, 303. doi.org/10.3389/fnhum.2015.00303
- Claudino, A. M., Van den Eynde, F., Stahl, D., Dew, T., Andiappan, M., Kalthoff, J., Schmidt, U., & Campbell, I. C. (2011). Repetitive transcranial magnetic stimulation reduces cortisol concentrations in bulimic disorders. Psychological medicine, 41(6), 1329–1336. doi.org/10.1017/S0033291710001881
- Jiang, B., & He, D. (2019, June 14). Repetitive transcranial magnetic stimulation (rtms) fails to increase serum brain-derived neurotrophic factor (BDNF). Neurophysiologie Clinique. https://www.sciencedirect.com/science/article/abs/pii/S0987705319300693
- Momi, D., Wang, Z., & Griffiths, J. D. (2023). TMS-evoked responses are driven by recurrent large-scale network dynamics. eLife, 12, e83232. doi.org/10.7554/eLife.83232
- Ferreira, F. F., Ribeiro, F. F., Rodrigues, R. S., Sebastião, A. M., & Xapelli, S. (2018). Brain-Derived Neurotrophic Factor (BDNF) Role in Cannabinoid-Mediated Neurogenesis. Frontiers in cellular neuroscience, 12, 441. doi.org/10.3389/fncel.2018.00441
- Cole, E. J., Phillips, A. L., Bentzley, B. S., Stimpson, K. H., Nejad, R., Barmak, F., Veerapal, C., Khan, N., Cherian, K., Felber, E., Brown, R., Choi, E., King, S., Pankow, H., Bishop, J. H., Azeez, A., Coetzee, J., Rapier, R., Odenwald, N., Carreon, D., … Williams, N. R. (2022). Stanford Neuromodulation Therapy (SNT): A Double-Blind Randomized Controlled Trial. The American journal of psychiatry, 179(2), 132–141. doi.org/10.1176/appi.ajp.2021.20101429
- Madore, M. R., Kozel, F. A., Williams, L. M., Green, L. C., George, M. S., Holtzheimer, P. E., Yesavage, J. A., & Philip, N. S. (2022). Prefrontal transcranial magnetic stimulation for depression in US military veterans - A naturalistic cohort study in the veterans health administration. Journal of affective disorders, 297, 671–678. doi.org/10.1016/j.jad.2021.10.025
- Hordacre, B., Comacchio, K., Williams, L., & Hillier, S. (2021). Repetitive transcranial magnetic stimulation for post-stroke depression: a randomised trial with neurophysiological insight. Journal of neurology, 268(4), 1474–1484. doi.org/10.1007/s00415-020-10315-6
- Croarkin, P. E., Elmaadawi, A. Z., Aaronson, S. T., Schrodt, G. R., Jr, Holbert, R. C., Verdoliva, S., Heart, K. L., Demitrack, M. A., & Strawn, J. R. (2021). Left prefrontal transcranial magnetic stimulation for treatment-resistant depression in adolescents: a double-blind, randomized, sham-controlled trial. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology, 46(2), 462–469. doi.org/10.1038/s41386-020-00829-y
- Rossi, S., Hallett, M., Rossini, P. M., Pascual-Leone, A., & Safety of TMS Consensus Group (2009). Safety, ethical considerations, and application guidelines for the use of transcranial magnetic stimulation in clinical practice and research. Clinical neurophysiology: official journal of the International Federation of Clinical Neurophysiology, 120(12), 2008–2039. doi.org/10.1016/j.clinph.2009.08.016
- Del Casale, A., Sorice, S., Padovano, A., Simmaco, M., Ferracuti, S., Lamis, D. A., Rapinesi, C., Sani, G., Girardi, P., Kotzalidis, G. D., & Pompili, M. (2019). Psychopharmacological Treatment of Obsessive-Compulsive Disorder (OCD). Current neuropharmacology, 17(8), 710–736. doi.org/10.2174/1570159X16666180813155017
- Roth, Y., Tendler, A., Arikan, M. K., Vidrine, R., Kent, D., Muir, O., MacMillan, C., Casuto, L., Grammer, G., Sauve, W., Tolin, K., Harvey, S., Borst, M., Rifkin, R., Sheth, M., Cornejo, B., Rodriguez, R., Shakir, S., Porter, T., Kim, D., … Zangen, A. (2021). Real-world efficacy of deep TMS for obsessive-compulsive disorder: Post-marketing data collected from twenty-two clinical sites. Journal of psychiatric research, 137, 667–672. doi.org/10.1016/j.jpsychires.2020.11.009
- Leocani, L., Dalla Costa, G., Coppi, E., Santangelo, R., Pisa, M., Ferrari, L., Bernasconi, M. P., Falautano, M., Zangen, A., Magnani, G., & Comi, G. (2021). Repetitive Transcranial Magnetic Stimulation With H-Coil in Alzheimer's Disease: A Double-Blind, Placebo-Controlled Pilot Study. Frontiers in neurology, 11, 614351. doi.org/10.3389/fneur.2020.614351
- Trung, J., Hanganu, A., Jobert, S., Degroot, C., Mejia-Constain, B., Kibreab, M., Bruneau, M. A., Lafontaine, A. L., Strafella, A., & Monchi, O. (2019). Transcranial magnetic stimulation improves cognition over time in Parkinson's disease. Parkinsonism & related disorders, 66, 3–8. doi.org/10.1016/j.parkreldis.2019.07.006
- Ameis, S. H., Blumberger, D. M., Croarkin, P. E., Mabbott, D. J., Lai, M. C., Desarkar, P., Szatmari, P., & Daskalakis, Z. J. (2020). Treatment of Executive Function Deficits in autism spectrum disorder with repetitive transcranial magnetic stimulation: A double-blind, sham-controlled, pilot trial. Brain stimulation, 13(3), 539–547. doi.org/10.1016/j.brs.2020.01.007
- Wang, S., Zhou, X., Huang, B., Wang, Z., Zhou, L., Wang, M., Yu, L., & Jiang, H. (2016). Noninvasive low-frequency electromagnetic stimulation of the left stellate ganglion reduces myocardial infarction-induced ventricular arrhythmia. Scientific reports, 6, 30783. doi.org/10.1038/srep30783
