As if from the pages of science fiction, the field of neuroprosthetics has exploded, reshaping what was thought possible. Such devices have allowed people to control the movement of robotic limbs through a computerized route rather than physical, muscular means. In 2012, Cathy Hutchinson, a quadriplegic woman who suffered a brainstem stroke, was able to feed herself with a DEKA prosthetic arm using her mind [1].
One of the major challenges of developing this technology for clinical use is that the number of signals received by these neuroprosthetic devices diminishes over a period of months [2]. In a study published in 2003 by Dr. Miguel Nicolelis of Duke University, 40% of recording electrodes stopped functioning within 18 months [4]. Another study by Drs. Patrick J. Rousche and Richard A. Normann showed that, initially only 7 of 11 electrodes could record and after 5 months, that number decreased to 4 of 11 [5].
Current research is aimed at finding ways to keep these devices functioning as well as possible for as long as possible. This means that fewer costly surgeries are required to replace the devices. Should technology and current methods continue to advance, humans may one day no longer need to fear the loss of motor function associated with various strokes and traumas. Thus, for Hutchinson and others like her, understanding and overcoming the decline in electrical function of neuroprosthetics is the first, critical step towards a more comfortable future.
Explanation
Studies have suggested this gradual reduction in functionality can be blamed on the brain’s immune response to foreign objects [2][3]. These immune responses come in two major varieties: an initial acute response to the implantation, and a long-term chronic response. When brain tissue is punctured, the implanted device will unavoidably strike capillaries, which are no more than 60 micrometers apart (about the diameter of a human hair). Part of the initial response is due to blood cells, activated platelets, clotting factors and inflammatory factors leaked from broken vessels. The larger the blood vessel that is struck, the greater these blood components contribute to the immune response.
In addition to the blood’s immune response, brain cells also carry out their own responses. Microglia, the cells involved in the brain’s immune response, will try, in an effort to protect the brain, to engulf the device. Because the device is too large for a single cell to envelop, a sheath of microglial cells forms around the device. Some studies have shown that microglia may even fuse together to form multi-nucleated large bodies. These bodies can appear as early as 18 days-post-implant [9] and have been found on silicon electrodes [10]. When microglia are unable to consume foreign objects, they enter a state known as “frustrated phagocytosis**” [2], in which they persistently release neurotoxic substances.
Killing neurons is one way microglia contribute to the loss of neuronal signals. As part of the immune response, microglia also send out pro-inflammatory cytokines to recruit astrocytes and other microglia, which eventually migrate to the injury site and congregate around the device. These cells might displace local neurons [10] further contributing to the reduction of signals over time. The proliferation of astrocytes and microglia around the device constitutes the chronic response [2].
One proposed theory for the chronic response involves the encapsulation of microglia and astrocytes around the implanted device [2][11][12][13][14]. It is commonly observed that astrocytes and microglia will form a layer of cells surrounding the implant, known as a “glial scar” [2]. Though, as these cells are actually not all dead, the area around the implant is not considered true scar tissue [6]. Regardless of whether these cells are dead or alive, their bilayer membranes will act as capacitors, storing electrical signals. This high concentration of cell membranes around the implant, in effect, decreases the amount of electrical signals that reach the electrodes, which reduces the functionality of the device. Over time, these implants receive only a fraction of the original signals.
The design of implant devices determines its “bio-compatibility”, which is the extent of tissue response to the device. Researchers have explored the use of plastics, polymers, ceramics, and glass, to reduce the severity of the initial and chronic responses. Microwire electrode arrays, as used by Dr. Nicolelis, can be made of conducting metals such as platinum, gold, or even stainless steel [2]. The next generation of recording devices, however, seems to be silicon-based electrode arrays [2].
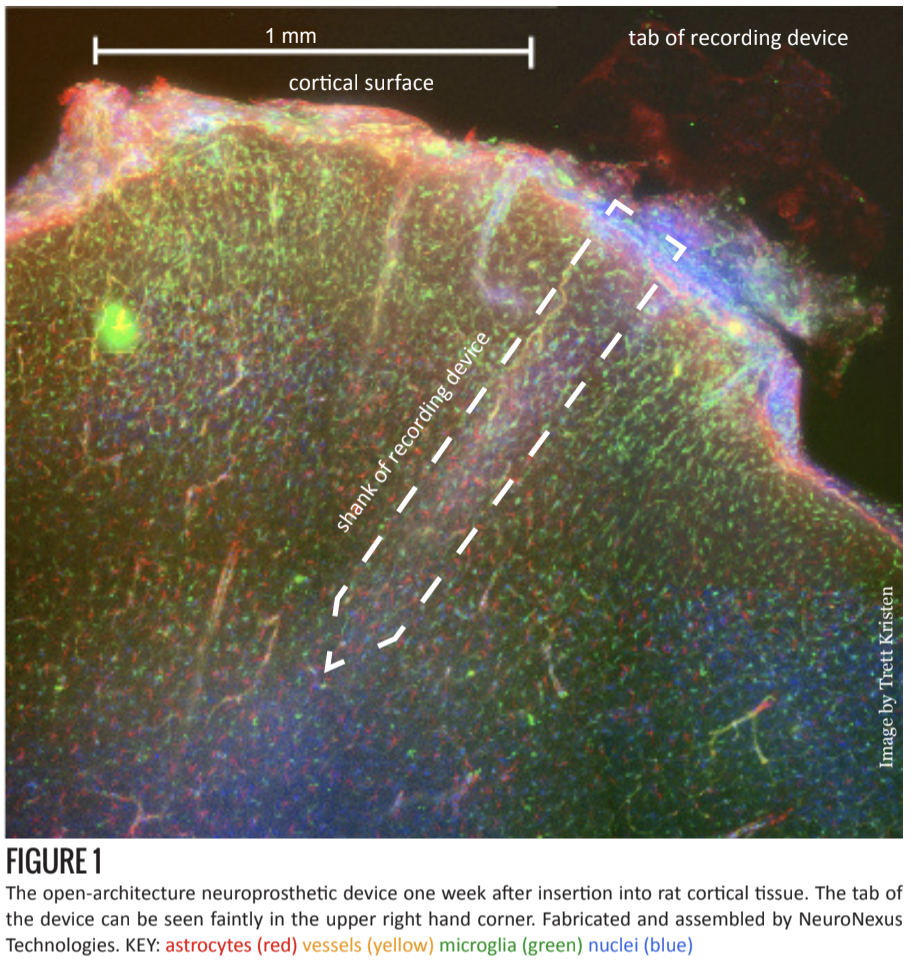
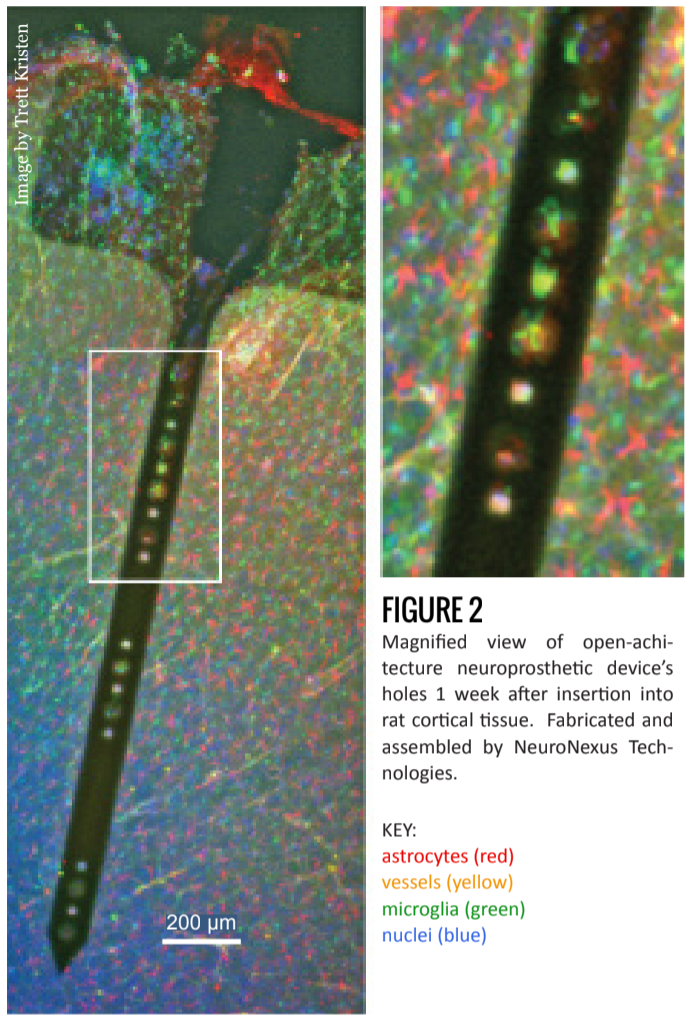
In addition to experimenting with materials, researchers have also tried using various anti-inflammatory drugs [7] and controlled time-release systems [8]. Currently in testing at the Shain lab at Seattle Children’s Research Institute, is the introduction of holes to the shank of the device (figures 1 and 2) [6].
Hypothesis
Dr. William Shain has hypothesized that astrocytes play a major role in the chronic immune response. Astrocytes are the most prolific of the cells in the human brain, comprising 30-65% of all glial cells in the central nervous system [15]. They support neural function by performing many different duties, one of which is absorbing excess potassium ions from the extracellular space [2]. During an action potential neurons release potassium into the extracellular space, where, without astrocyte intervention, the ions would accumulate and inhibit neuronal function (For more information regarding action potentials).
Astrocytes form extensive physical networks with each other through gap junctions. Ions absorbed by astrocytes are diffused and distributed throughout this network. However, as Dr. Shain suggests, when foreign objects, such as neuroprosthetic implant devices, destroy these intercellular connections, they interrupt the ions’ routes of diffusion. As a result, ion concentrations build up inside the astrocytes, and osmosis causes the cells to swell. Under stress, astrocytes will enter a “reactive” mode in which they exhibit increased migration, proliferation, and matrix production [16]. They will also increase production of molecules that signal for other microglia and other astrocytes. This results in further congregation of cells around the device in the chronic response.
Building on the hypothesis that a chronic response is aggravated by the disruption of the astrocyte network, Dr. Shain is testing devices that may allow the rebuilding of such networks. The project seeks to facilitate ion diffusion by allowing astrocytes to re-form connections through, and not just around, the device. This is done by building electrodes with 20 micrometer-wide holes throughout the device shank. In the rat cortex, an astrocyte’s processes are as long as 30 micrometers, and the hole is 15 micrometers thick. Two astrocytes, then, can theoretically form a 60 micrometer-long connection body-to-body.
The Future
What researchers want to know is how proteins, cells, and cell patterns change in response to implantation. Tissue samples, which may range from 100 to 300 microns thick, are captured in 3D images using spinning-disk confocal microscopy. Using a program called FARSIGHT, the cell count and cell locations can then be recorded from these 3D images, resulting in usable, quantitative data. Through the analyses of these data, researchers can better understand the brain’s responses to implantation.
This ongoing research into neuroprosthetic devices will further aid scientists in the quest to explain the physiology of the chronic response. These discoveries will ultimately benefit patients such as Cathy Hutchinson, who suffer from severe forms of paralysis, by allowing implanted devices to function longer and continue to improve the patients’ day-to-day lives.