Cells first were made visible with very simple microscopes designed several centuries ago. The evolution of microscopic techniques has since allowed for highly detailed image capture at nearly 10,000,000x magnification. Microscopes are used for characterizing phenotypes, localizing protein, and everything in between. And although microscopy is an integral component of much scientific research, the principles behind these techniques are not well understood by many users.
The Basics: Compound Light Microscopes
Compound light microscopes, although the least complex, provide the fundamentals necessary to understand more intricate microscopy techniques. The sample is placed just beyond the convergence point for the objective lens. Light is refracted through the convex objective lens to converge at a much greater length than the original distance between the object and objective lens. The ratio of these two distances provides the magnification of the object. Because this positive convex lens also inverts the image, an additional eyepiece lens acts to revert the image (figure 1). The user can focus the image by adjusting the distance between the two lenses. The source of light can be from below (for transmitted light through a translucent object) or above (for reflected light from an opaque object). These microscopes are typically of low magnification, but are commonly used due to rapidity, ease of sample preparation, and simplicity of use.
Fluorescent Microscopy: Confocal and Two Photon Microscopes
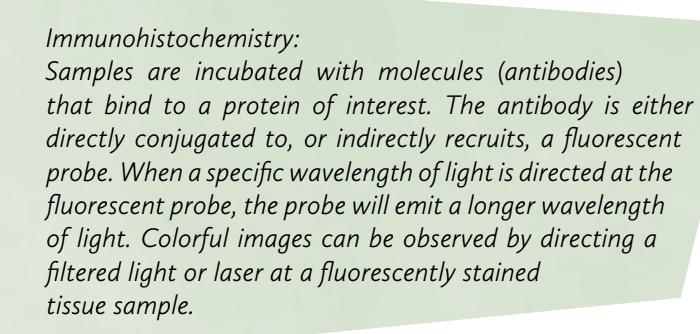
Fluorescent microscopes, although fundamentally similar to compound light microscopes, employ complex principles to image specific components of cell or tissue systems. Controlled light, such as a weak laser, is directed to the sample using a dichroic beam-splitting mirror. Sample preparation often involves Immunohistochemistry to fluorescently label desired cellular components. If the laser excites a fluorescent probe, the emitted wavelength passes through the dichroic mirror and is seen through the eyepiece. This type of light filtration allows for the manipulation of single wavelength light paths.
Confocal microscopy is a modified technique used to obtain images from a single horizontal plane, usually to localize proteins or other molecules. By imaging one horizontal plane at a time, the overlaying signals on the vertical axis do not merge, separating overlaying clusters of fluorescent probes. Confocal microscopes are capable of only allowing light from a single plane by screening unwanted light through a pinhole (figure 2). Similarly to the light path of a compound microscope, the image converges to a point. The microscope must perform a row-by-row raster scan, making confocal microscopy a very time-intensive imaging technique. For confocal microscopy to thus be applicable to live imaging, spinning disk techniques are used to capture separate images from multiple areas in parallel.
Additionally, there is the potential to photobleach the sample, rendering the fluorescent probe, and thus image acquisition, useless. An alternative to confocal microscopy that reduces photobleaching is two photon microscopy. Although this lacks the temporal resolution of spinning disk confocal microscopy, two photon microscopy also provides better spatial resolution. This technique employs two sources of light, each composed of half the energy necessary to excite the fluorescent probe. When the two beams of light cross at a point, the region gains the energy sufficient for fluorescence emission.
Imaging Synapses: Electron Microscopes
There are two types of electron microscopes: transmission and scanning electron microscopes. Transmission electron microscopes capture electrons after they pass through the sample, while scanning electron microscopes capture the electrons reflected from the surface of the sample.
The electron beam is made by pulling negatively charged particles to a positively charged plate (anode), and focusing the spray with a negatively charged plate (cathode). This spray of electrons is further controlled to a beam through the use of electromagnetic lens condensers (figure 3). When the electrons hit the sample, they interact in a variety of ways with the particles present in the sample. The detector captures these interactions and software programs analyze the results to create image for the user. Although electron microscopy provides high levels of detail on topographical structure, sample preparation takes time and live imaging is not an option.
References
- Paul A. Midgley, The principles of microscopy, Materials Today, Volume 9, Issue 10, October 2006, Page 57, ISSN 1369-7021, http://dx.doi.org/10.1016/S1369-7021(06)71657-1.
- Semwogerere, D., & Weeks, E. R. (2005). Confocal Microscopy. Encyclopedia of Biomaterials and Biomedical Engineering, 1–10. http://doi.org/10.1081/E-EBBE-120024153
- Dubochet, Jacques et al. “Cryo-Electron Microscopy of Vitrified Specimens.” Quarterly Reviews of Biophysics 21.02 (1988): 129–228. Cambridge Journals Online. Web. 22 Aug. 2015.