Until the 1950s, common theories of sleep involved the brain shutting down, or powering off, similar to a car engine. Research since then, however, has shown high levels of activity in the human brain for the duration of the periods in which it is asleep, especially in the hypothalamus [1].
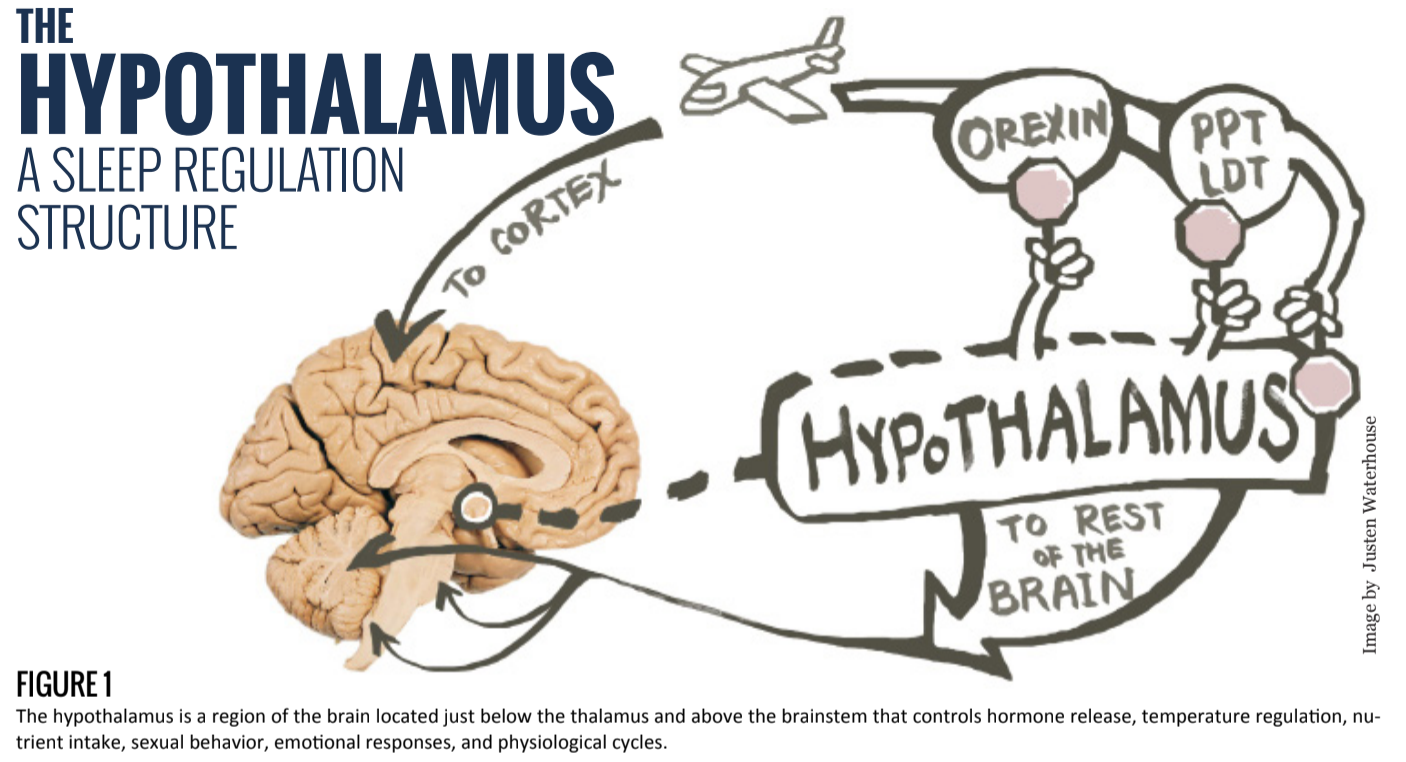
The hypothalamus is a region of the brain located just below the thalamus and above the brainstem that controls hormone release, temperature regulation, nutrient intake, sexual behavior, emotional responses, and physiological cycles.
Interestingly, the hypothalamus is inhibited by the same arousal structures it inhibits during sleep [2] (see Figure 1). This creates a positive feedback loop, which also explains the swift transition from wakefulness to sleep and vice versa. As such, it would be more accurate to portray its activity as a “flip-flop” switch, in which both states indicate that the brain is “on” but has alternating function.
As a central part of the nervous and endocrine system, the hypothalamus, which broadly receives and distributes information, is well suited for regulating sleep. Most importantly for the flip-flop switch, the hypothalamus contains an ascending pathway to the thalamus itself and activates thalamic relay neurons, allowing the region to transmit information to the cerebral cortex and the rest of the brain.
Within the hypothalamus resides the ventrolateral pre-optic (VLPO) area, which is a structure crucial for flipping the brain from the waking to sleeping state. An active, functioning VLPO is what maintains the brain in either condition. This is done mainly through the projection of gamma-aminobutyric acid (GABAergic) cells [3].
During periods of wakefulness, an acetylcholine-generating cell group referred to as the pedunculopontine and laterodorsal tegmental nucleus (PPT/LDT) relays information between the thalamus and cerebral cortex. It has been shown that this group has a high activation rate during wakefulness, which ceases during REM sleep [4]. The PPT/LDT cells are activated by orexin-producing neurons, and additionally inhibit the VLPO, which, while unsuppressed, blocks both PPT/LDT activity as well as orexin production [5]. As a result, orexin drives the maintenance of wakefulness and therefore the continuous inhibition of VLPO during the day. These neurons’ activations are maintained by the human brain’s circadian cycles and photoresponse mechanisms [3].
Because of the mutual inhibition, the activity of either PPT/LDT or VLPO will suppress activity from the other circuit and therefore ensure its own function, making clear the distinct shift in the human brain into and out of a sleeping state.
The reciprocity of this system ensures there is no gradual shift from the sleep state to the wakeful one and vice versa, another reason why a better model for this transition would be the “flip-flop” switch: instead of on and off states, the switch indicates only the swift transition between awake and asleep, making no suggestion that the brain is inactive in either state.
Because the hypothalamus closely regulates this flip-flop switch, any harm caused to this area can dramatically affect sleep cycles. The dependency of the neurotransmitters’ activations on a functioning VLPO leaves little room for error. If insufficient GABAergic cells are activated by the VLPO, there will not be enough impetus to push the brain from one state to the other. It is not surprising then, that research has shown a link between damage to the hypothalamus and sleep disorders. Weakening either side of the flip-flop switch would lead to its malfunction and the creation of an undesirable transition point in between the two states [3].
In any case, it is considered evolutionarily beneficial for animals to have sudden sleep-wake transitions. Spending periods in a half-asleep, half-awake state would decrease alertness and in many cases be dangerous as it increases risk from predators.
Indeed, the inability to control this transition is commonly seen as a symptom of narcolepsy, a neurodegenerative disease. The benefits of sleep would be diminished as well, further demonstrating little benefit to a prolonged transition state [5].
In properly functioning brains, the regulation of the VLPO through circadian cycles ultimately drives the switch. In short, the flip-flop model of sleep explains many of the ways in which sleep is mediated as well as why disorders arise when the cycle is damaged—thus providing a base and framework for further testing and analysis.