Introduction
In Denmark, researchers at the University of Copenhagen have identified specific genetic predispositions in mice that eventually impair the development of oligodendrocytes, crucial cells that produce the myelin sheath [1]. At the University College Cork in Ireland, scientists used mice to find a link between changes in gut bacteria and the display of behaviors characteristic of anxiety and depression [2]. At UC Irvine, researchers implanted human microglia into mouse brains, then replicated the environmental characteristics of Alzheimer’s [3].
Whether through visualizing the activity of the mouse brain or implanting specially-designed neural tissue, an important component of each project was the use of mice. Each lab used mice as a model organism for a human disease or condition, then successfully extrapolated their findings towards humans. Although the topics of the research done at each lab are interesting in their own right, the success of each of the three projects acts as a reflection on the state of current neuroscience research. Namely, research in realms where it is difficult, unethical, or impractical to experiment on humans is often conducted on mice.
In basic science research, the commonality of mouse models is no surprise. Evolutionarily and genetically, mice share similarities with humans, making many human diseases and conditions replicable in a lab setting. Perhaps more importantly, mice are convenient to use, as they are cheap to maintain and have short lifespans. These characteristics make mice desirable not only in the field of neuroscience, but in almost all biological research. A recently published article estimates that more than 110 million rats and mice are killed each year in US laboratories alone [4]. Without the contributions of mice, our understanding of the human brain would be far more primitive, and the aforementioned discoveries would have been impossible.
It might sound surprising that our foundational knowledge of human brain function comes as much from mouse brains as from human brains. This reliance has raised questions about the potential problems of using mice as model organisms for the human brain. Modeling human disease, especially when it comes to correlating the structures of mouse and human brains, is an imperfect practice. Human and mice organs share many similarities, but the particular complexities of the brain introduce differences.
In practice, the imperfection of the mouse brain as an analog for the human brain has led to tangible failures in the development of drugs for the central nervous system (CNS). Compared to other drugs, drugs targeting the central nervous system (CNS) have higher failure rates, longer approval times, and higher developmental costs [5]. Between 1995 and 2007, FDA approval rates for CNS drugs (6.2%) were less than half the approval rates for non-CNS drugs (13.3%) [5]. Low CNS drug approval rates have caused a reevaluation of the rodent-reliant paradigm. Ongoing research has revealed both previously unknown differences between the mouse/human brain, and generated proposals for new ideas and solutions. Though modern neuroscience treatments are built on the backs of millions of slaughtered mice, this new research hints at why mouse models for the brain so often fail and why a different approach may yield more success. But before we delve into some of these new approaches, it is worth understanding why mice have been and will continue to be essential for neuroscience research.
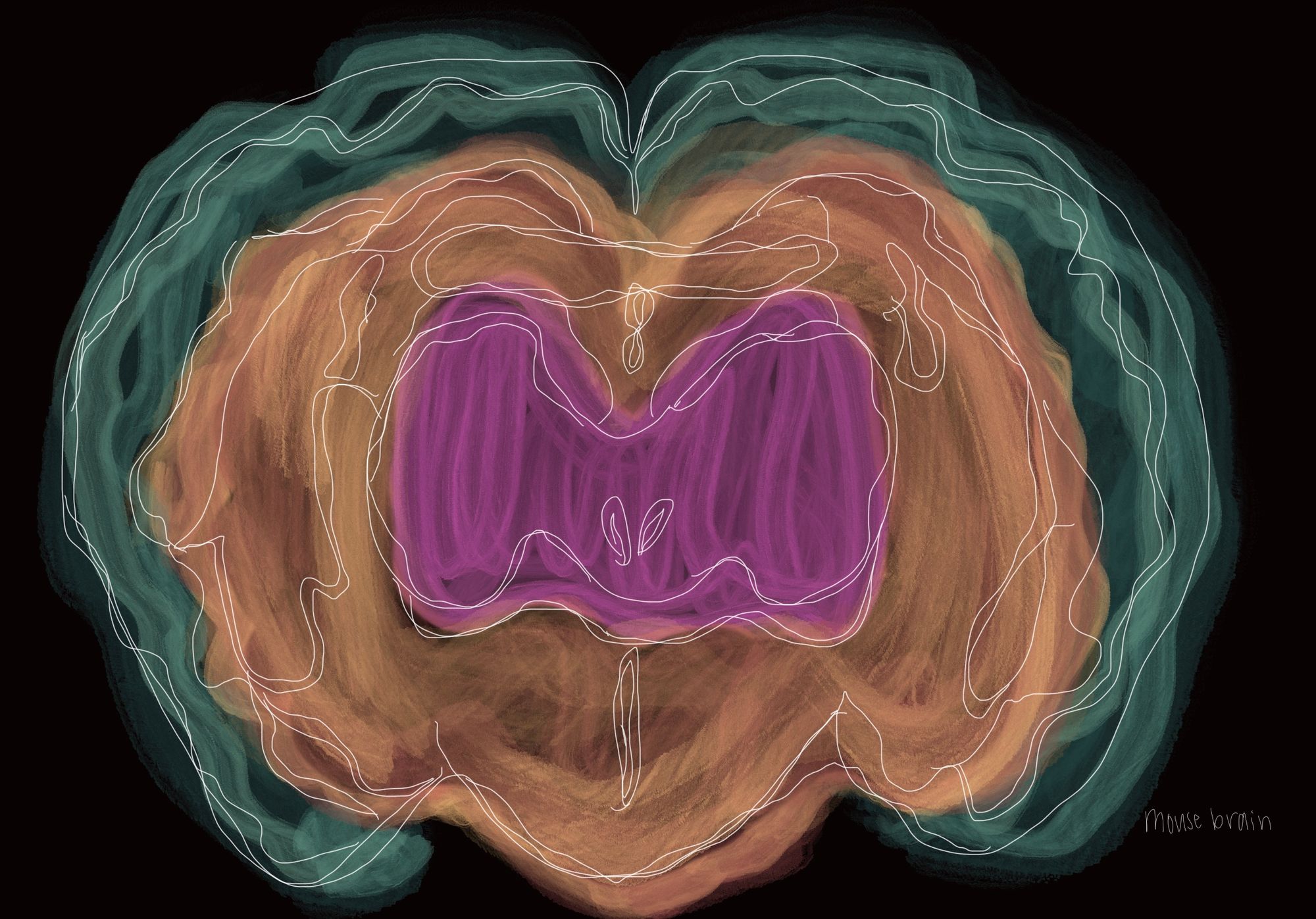
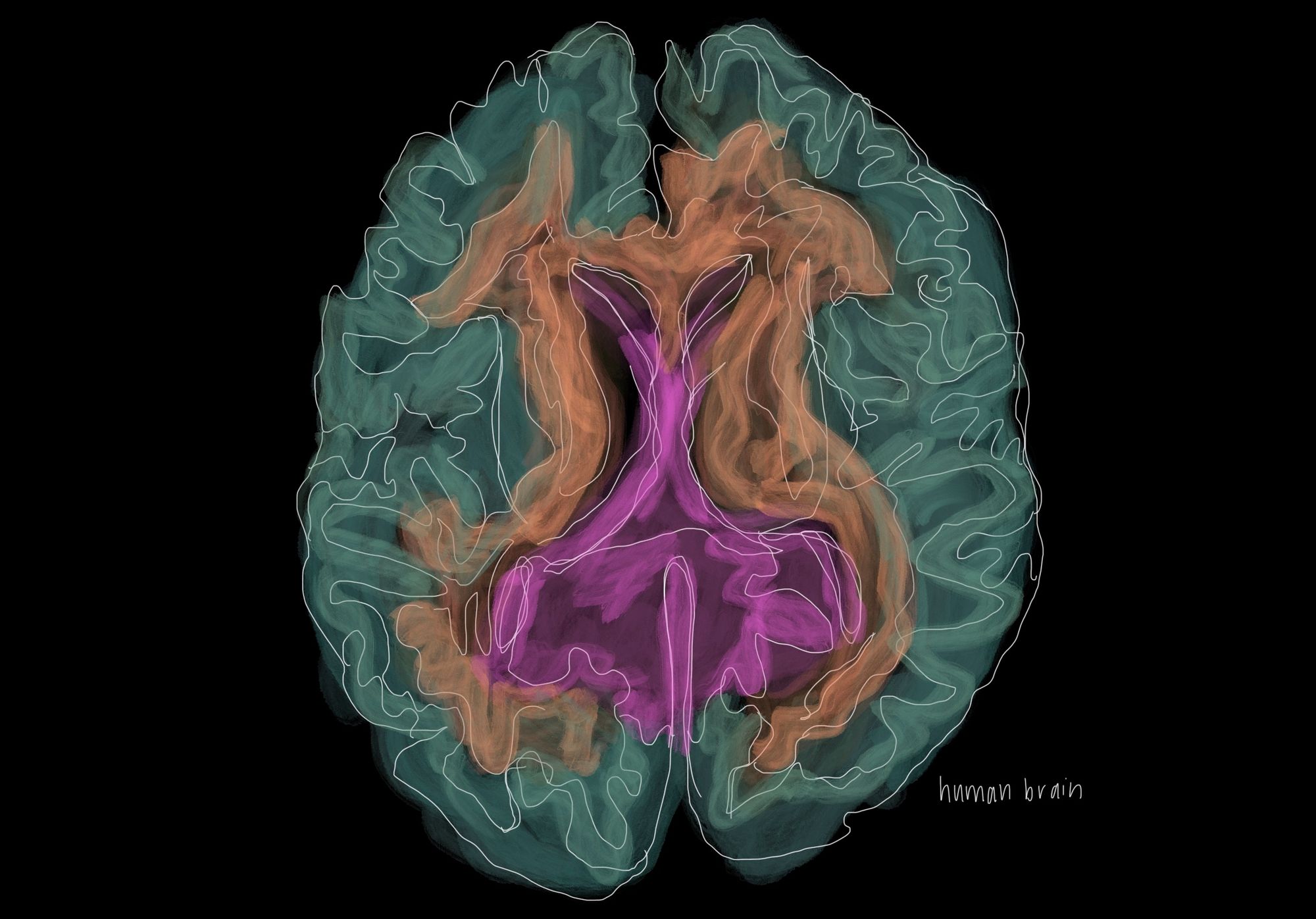
Why Rodents Are Used as Models for the Human Brain
It is not as if mice and rats were arbitrarily chosen for use in neuroscience. To accurately replicate human diseases, the anatomical similarities between mice and humans are extremely helpful. Historically, the use of mouse models were justified by observations of the conserved nature of the mouse and human brain [6]. This means that the brain tissue of the two are similar when looked at under a microscope. Recent research generally agrees with these historical findings. Closer examinations into the visual cortex of both primates and mice have found important similarities in architecture. For one, the visual cortex of the mouse has similar distance rules, meaning that areas in close proximity are more interconnected than those further apart [7]. Additionally, the extrastriate cortex in mice, similar to humans, processes visual information through two separate pathways [8]. This two-stream system separately processes the “what” (object identification) and the “where” (location) of visual input, and is similar to the way humans process vision [9]. These two findings demonstrate that shared key features exist so that mice remain useful in the study of visual systems.
On a larger scale, multiple studies have also found correlations in gene expression within mouse and human brains, showing additional similarities between the systems. At the Fred Hutchinson Research Center in Seattle, researchers compared the gene expression profiles of the two species [10]. In humans, the researchers examined three regions with differential gene expression in the brain (the motor cortex, caudate nucleus, and cerebellum), then identified the genes that were expressed specifically in these regions. In mice, the researchers conducted the same analysis. These researchers found that the genes expressed in the human brain regions were also expressed in the corresponding mouse brain regions. Furthermore, regionally-expressed genes tended to have greater similarities in nucleotide sequence than widely-expressed genes. Essentially, the characteristic genes that identify the different regions are generally conserved, both in terms of nucleotide sequence and expression [10]. A more recent study at the Allen Institute in Seattle used RNA sequencing to find a, “surprisingly well-conserved cellular architecture [in mice] that enable[d] matching of homologous types and predictions of properties of human cell types” [11]. These findings correlate with the findings of the Fred Hutch researchers. In combination, they show that regional gene expression is similar between the two species.
It is also important to note that use of mice is particularly popular due to a well-developed understanding of its genome as well as the availability of useful genetic tools to edit them. Of particular importance is the Cre/loxP system, a recombination system used to manipulate the gene expression of genetically modified mice, allowing for the deletion of a gene in specific tissues of a mouse [12]. In practice, neuroscience researchers have used Cre/loxP to visualize neurons using unique labels, furthering our understanding of the connections within a mouse’s nervous system. This recombination system, along with other strategies that ease gene editing and recombination, makes research with mice more efficient than with other mammalian species. For neuroscientists, these technological developments have been crucial in studying the function and interaction of complex cell types [13]. In practice, scientists find that the utility of these tools compensates for the physiological differences between mice and humans.
Problems with the Rodent Model – Both Old and New
A key difficulty in using mouse models is the accurate replication of human disease. For example, depression in humans is screened through a questionnaire about our behaviors, emotions, and motivations. When it comes to mice, how are scientists supposed to identify depression? As fun as it may be to imagine a mouse filling out a survey with a tiny clipboard and pen, in reality scientists identify depression in mice by observing their behavior in the forced-swim test (FST). In the FST, mice are plunged into a cylinder of water and tested for how long they struggle. If a mouse gives up quickly, it is said to be depressed [14]. First used in the 1970s, the FST was crucial to the development of selective serotonin reuptake inhibitors (SSRIs), a class of drugs which includes Prozac. For mice that demonstrated depressive behavior, SSRIs consistently improved the duration over which the mice struggled in the water.
The proliferation of the FST as a standard test for depression, despite its success in the development of SSRIs, is controversial. Its critics often point to RD Porsolt, the creator of the FST, who stated that the test could not be used as a direct model for depression since the dependent variable is “a response of the animal to the test, not a characteristic of the animal” [15]. In other words, the test measures behaviors that might be symptomatic of depression, but that does not necessarily mean that the observed behaviors are caused by depression. Other aspects of the FST are even more puzzling. For one, as measured by the FST, the behavior of mice given SSRIs changes within days [16]. In humans, SSRIs only reduce symptoms of depression after weeks or months of treatment. Furthermore, meta-analyses of the FST have found its results to have limited reproducibility across different labs, indicating that behavior during the FST is contingent on conditions within individual laboratories [17]. As a result of these concerns, the National Institute of Mental Healthno longer recommends the FST as a model of depression, and the pharmaceutical companies Roche, Janssen, and AbbVie have transitioned to other models of depression [14].
Amidst drug failures in neuroscience, the FST is only a small piece of a broader movement to reevaluate many of the prevailing behavioral models. Often, the problem with these models is not intrinsic to mice, but rather a result of poor experimental design and a tendency towards adhering to simpler experiments like the FST. In these cases, abandoning mice would be unnecessary. Simply redesigning these models may solve existing problems, and for depression, has already yielded promising results. For example, in the social defeat model, a smaller mouse is placed in a cage with a bigger mouse [17]. After ten days of fighting in which the smaller mouse is dominated, the smaller mouse shows decreased interest in sex, drinking sugar water, and social contact. Compared to the FST, these symptoms are more similar to those displayed by people with depression and are induced by chronic social stressors that parallel human experiences. In these mice, the symptoms are alleviated by “chronic, but not acute” administration of an antidepressant, matching what is observed in humans [18].
While mouse studies can be improved by changing behavioral models, new research has revealed fundamental cellular differences between the mouse and human brain. In a collaborative study at the University of Szeged in Hungary and the Allen Institute in Seattle, researchers identified a new type of brain cell – the “rosehip” neuron [19]. The rosehip neuron is found in humans, but not in mice. Although the exact function of these neurons is as of yet unclear, rosehip neurons are inhibitory, producing GABA, and are connected to pyramidal neurons that function as the primary excitation units of the prefrontal cortex. Because the function and dysfunction of inhibitory neurons are essential to diseases like schizophrenia, the presence of rosehip neurons suggests that the development of certain mental illnesses in humans is likely unique compared to other mammals [19].
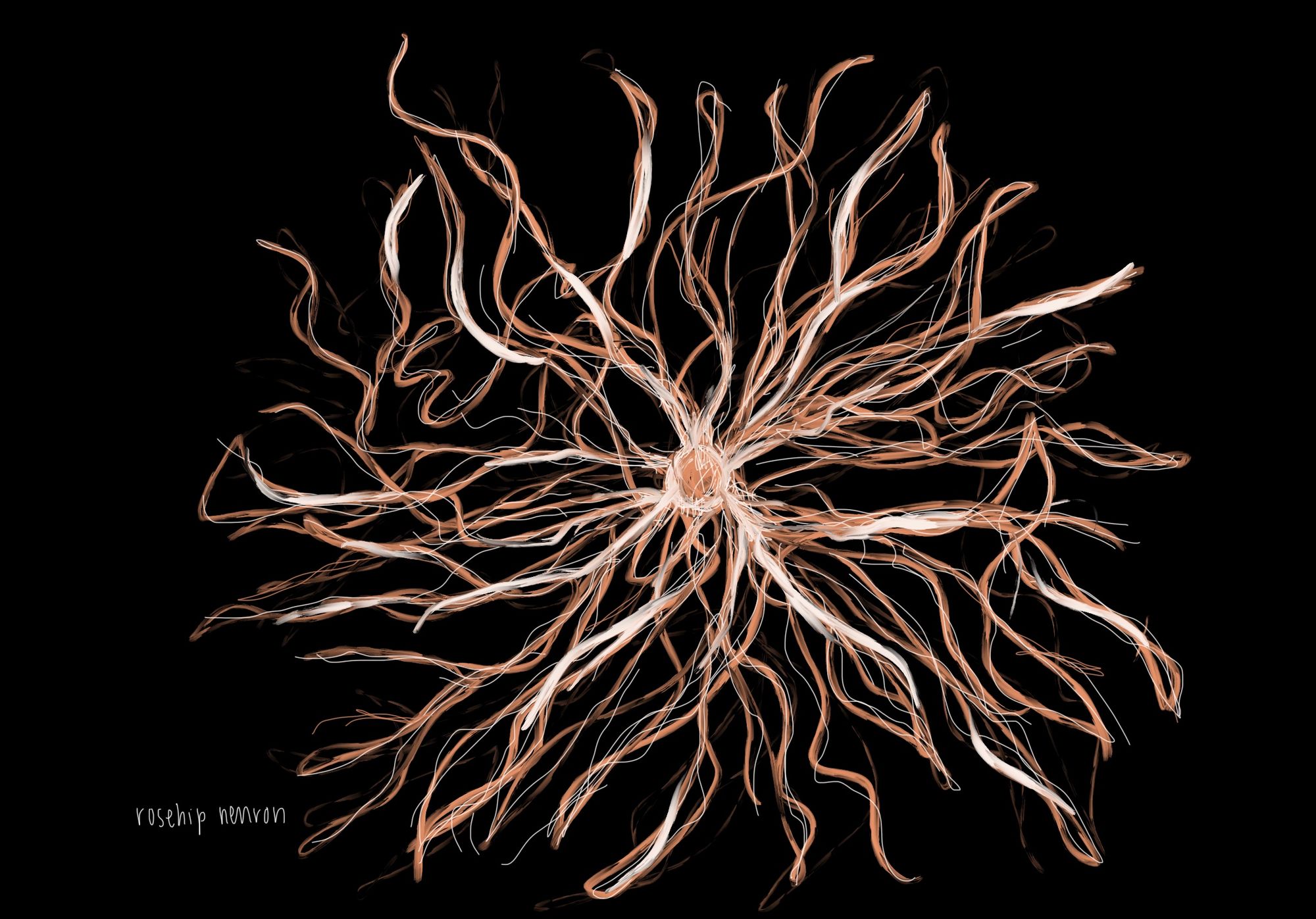
Additional research by the Allen Institute has found that, even among cell types that are found in both humans and mice, differential gene expression affects the function of the neurons [11]. Notably, the expression of genes that encode for serotonin receptors were highly divergent. As a result, a mouse’s serotonin receptors are not always found in the same cell types as they are in humans. Since abnormal serotonin function is linked to depression, anxiety, and other mood disorders, the differential expression of serotonin receptors may explain why SSRIs that treat disease in mice often fail in humans [19][20].
Regardless, research showing similarities between the brains of humans and mice confirms a fact of which scientists are already aware: mouse models work most of the time. The constant stream of new findings based on mouse research is a testament to this fact. Still, mouse models often fail. In these cases, the differences become more important, because they introduce unknown interactions and consequences that make the findings in mice less applicable to humans. As a result, the aforementioned research is crucial in understanding how these differences compromise generally successful models. With this knowledge, scientists can design new models that account for differences and yield results that are more directly applicable to humans.
Promising Research on New Models
Mouse models have been integral to our understanding of the human brain and the causes of neurological disorders. Even if mice are rarely perfect models, they are almost always good enough. Therefore, mice will continue to be used as models for the human brain. As our understanding of the brain continues to expand, the contributions of millions of mice will be forever invaluable.
Still, improvements to neuroscience models are always welcomed. Recent developments indicate that pluripotent stem cells, prized for their ability to differentiate into every possible cell type, may be the key to improving our understanding of the human brain. In 2007, Shinya Yamanaka pioneered a new method in which mature human skin cells could be induced to dedifferentiate back to pluripotent stem cells [21]. Because these induced pluripotent stem cells (iPSCs) are patient-specific and give rise to all other cell types, this new technique is broadly relevant to the study of diseases. iPSCs are of growing interest in neuroscience research. Although there are many different applications for stem cells, one recent development is their use in forming structures called organoids. Organoids are small three-dimensional clusters of cells that are derived from stem cells. Under appropriate conditions, these organoids can represent specific organs, with similar structures and developmental patterns [22]. As a result, there is potential for organoids to be used to improve our understanding of the human brain.
In the past, development of brain organoids in vitro has been severely limited by the lack of nutrients available to cells in the center of a growing organoid. In 2013, researchers in Vienna developed a new method that allowed brain organoids to expand to greater sizes and more closely resemble the cerebral cortex of an embryo [23]. Of particular importance was the use of a spinning bioreactor, which is able to provide more nutrients to cells within the organoid. These small organoids contain multiple brain-like regions and are useful for tracking how stem cells differentiate during embryonic development. However, it is important to note that these cerebral organoids are not yet great models of the brain. Formation of different regions is random, resulting in a lack of the structure and organization seen in a normal brain. The development of organoids is also constrained by the lack of important environmental signals received by the embryo during gestation.
Still, these organoid models have one clear advantage over in vivo mouse models. Namely, the structures and cell types unique to the human brain are expressed in these organoids, removing some of the confounding factors of using mouse models. Although mouse models are still more convenient and accurate in most use cases, researchers in Vienna have already used the organoid model to successfully model disease. Microcephaly, a disease in which a baby grows an abnormally small head, can be caused by several genetic mutations. In mice, however, the same genetic mutations fail to produce a similar phenotype in mice. In the iPSC organoid model, the genetic mutations resulted in a large decrease in the population of stem cells that differentiate in neurons and glia [23]. Essentially, in recreating the disease, the organoid model succeeded where the mouse model failed.
Distinct as they may seem, the organoid and mouse models are not necessarily divergent. In 2018, researchers at the Salk Institute in San Diego successfully transplanted human brain organoids into the brain of adult mice [24]. In the mice, the organoids integrated into the mouse brain, where they were supplied with blood and nutrients so they could survive and continue developing. The organoids even formed synapses with the mouse brain cells, creating connections between the human and mouse portions of the brain. While bioethicists raise concerns about how implanted human brain cells could affect the consciousness of mice, the technique is useful because it allows for greater flexibility in testing human cells under actual physiological conditions. Even as neuroscience research advances, the integration of mice with newly designed models means that mice will continue to be an integral part of the quest to understand the human brain.
Conclusion
While animal research has undoubtedly contributed to the advancement of human health, ample concerns remain about the ethics of animal research. Everyone is aware of PETA, the single organization that is perhaps the most vocal about abolishing the use of lab animals. Yet what PETA is advocating for is hardly a fringe position. According to Gallup, around 40% of US adults find medical testing on animals to be unethical. Inevitably, these concerns have affected the way biological research is conducted [25]. The advocacy of both scientific institutions and the scientists within them have resulted in regulations and guidelines on ethical treatment of animals. Described in 1959, the “Three Rs” of Replacement, Reduction, and Refinement are now commonplace among both principle and practice. More recently, institutions are required to have Institutional Animal Care and Use Committees (IACUC), which ensure that protocols abide by the law. Regardless, each life lost in the lab is still a life lost. For this reason, new models which promise to reduce the number of animals used in the lab are of high interest to scientists. That is not to say that these models do not present concerns of their own. Organoids, for example, raise ethical questions about the identity of mice when implanted with human tissue. But if refined, these new models still have the potential to greatly reduce the ethical burden inherent within animal research.
Outside of the Institute of Cytology and Genetics in Novosibirsk, there is a statue of an anthropomorphized mouse, wearing glasses and knitting a strand of DNA. Standing at 2.5 meters high, the statue was built to commemorate the sacrifice of mice in genetics research used in advancing medicine and curing diseases. This sentiment extends beyond genetics researchers in Russia. For decades, Asian research institutions, particularly those in Japan, have held yearly tributes to research animals, a practice that has since been adopted by the NIH and other Western research institutions [26]. But for all the pain and suffering that mice have undergone at the hands of researchers, there is no doubt that mouse research has yielded tangible benefit, whether in the form of cancer drugs, HIV antiretrovirals, or COVID-19 vaccines. Still, if new models can reduce the number of mice used in lab research, then they will surely be welcomed. For example, organoids have the potential to reduce animal suffering in many stages of research. But until radically different methods are commonplace, mice and other lab animals will remain essential to research for the foreseeable future. In that time, it is vital that we remember the contributions that mice have already made and minimize the costs that future contributions incur.
References
- Windrem, M. S., Osipovitch, M., Liu, Z., Bates, J., Chandler-Militello, D., Zou, L., Munir, J., Schanz, S., McCoy, K., Miller, R. H., Wang, S., Nedergaard, M., Findling, R. L., Tesar, P. J., & Goldman, S. A. (2017). Human iPSC Glial Mouse Chimeras Reveal Glial Contributions to Schizophrenia. Cell stem cell, 21(2), 195–208.e6. https://doi.org/10.1016/j.stem.2017.06.012
- Hoban, A. E., Stilling, R. M., M Moloney, G., Moloney, R. D., Shanahan, F., Dinan, T. G., Cryan, J. F., & Clarke, G. (2017). Microbial regulation of microRNA expression in the amygdala and prefrontal cortex. Microbiome, 5(1), 102. https://doi.org/10.1186/s40168-017-0321-3
- Hasselmann, J., Coburn, M. A., England, W., Figueroa Velez, D. X., Kiani Shabestari, S., Tu, C. H., McQuade, A., Kolahdouzan, M., Echeverria, K., Claes, C., Nakayama, T., Azevedo, R., Coufal, N. G., Han, C. Z., Cummings, B. J., Davtyan, H., Glass, C. K., Healy, L. M., Gandhi, S. P., Spitale, R. C., … Blurton-Jones, M. (2019). Development of a Chimeric Model to Study and Manipulate Human Microglia In Vivo. Neuron, 103(6), 1016–1033.e10. https://doi.org/10.1016/j.neuron.2019.07.002
- Carbone L. (2021). Estimating mouse and rat use in American laboratories by extrapolation from Animal Welfare Act-regulated species. Scientific reports, 11(1), 493. https://doi.org/10.1038/s41598-020-79961-0
- Gribkoff, V. K., & Kaczmarek, L. K. (2017). The need for new approaches in CNS drug discovery: Why drugs have failed, and what can be done to improve outcomes. Neuropharmacology, 120, 11–19. https://doi.org/10.1016/j.neuropharm.2016.03.021
- Herculano-Houzel, S., Catania, K., Manger, P. R., & Kaas, J. H. (2015). Mammalian Brains Are Made of These: A Dataset of the Numbers and Densities of Neuronal and Nonneuronal Cells in the Brain of Glires, Primates, Scandentia, Eulipotyphlans, Afrotherians and Artiodactyls, and Their Relationship with Body Mass. Brain, behavior and evolution, 86(3-4), 145–163. https://doi.org/10.1159/000437413
- Laramée, M. E., & Boire, D. (2015). Visual cortical areas of the mouse: comparison of parcellation and network structure with primates. Frontiers in neural circuits, 8, 149. https://doi.org/10.3389/fncir.2014.00149
- Wang, Q., Sporns, O., & Burkhalter, A. (2012). Network analysis of corticocortical connections reveals ventral and dorsal processing streams in mouse visual cortex. The Journal of neuroscience : the official journal of the Society for Neuroscience, 32(13), 4386–4399. https://doi.org/10.1523/JNEUROSCI.6063-11.2012
- Milner AD. How do the two visual streams interact with each other?. Exp Brain Res. 2017;235(5):1297-1308. doi:10.1007/s00221-017-4917-4
- Strand, A. D., Aragaki, A. K., Baquet, Z. C., Hodges, A., Cunningham, P., Holmans, P., Jones, K. R., Jones, L., Kooperberg, C., & Olson, J. M. (2007). Conservation of regional gene expression in mouse and human brain. PLoS genetics, 3(4), e59. https://doi.org/10.1371/journal.pgen.0030059
- Hodge, R. D., Bakken, T. E., Miller, J. A., Smith, K. A., Barkan, E. R., Graybuck, L. T., Close, J. L., Long, B., Johansen, N., Penn, O., Yao, Z., Eggermont, J., Höllt, T., Levi, B. P., Shehata, S. I., Aevermann, B., Beller, A., Bertagnolli, D., Brouner, K., Casper, T., … Lein, E. S. (2019). Conserved cell types with divergent features in human versus mouse cortex. Nature, 573(7772), 61–68. https://doi.org/10.1038/s41586-019-1506-7
- Gurumurthy, C. B., & Lloyd, K. (2019). Generating mouse models for biomedical research: technological advances. Disease models & mechanisms, 12(1), dmm029462. https://doi.org/10.1242/dmm.029462
- Tsien J. Z. (2016). Cre-Lox Neurogenetics: 20 Years of Versatile Applications in Brain Research and Counting…. Frontiers in genetics, 7, 19. https://doi.org/10.3389/fgene.2016.00019
- Reardon S. (2019). Depression researchers rethink popular mouse swim tests. Nature, 571(7766), 456–457. https://doi.org/10.1038/d41586-019-02133-2
- Molendijk, M. L., & de Kloet, E. R. (2015). Immobility in the forced swim test is adaptive and does not reflect depression. Psychoneuroendocrinology, 62, 389–391. https://doi.org/10.1016/j.psyneuen.2015.08.028
- Yankelevitch-Yahav, R., Franko, M., Huly, A., & Doron, R. (2015). The forced swim test as a model of depressive-like behavior. Journal of visualized experiments : JoVE, (97), 52587. https://doi.org/10.3791/52587
- Kara, N. Z., Stukalin, Y., & Einat, H. (2018). Revisiting the validity of the mouse forced swim test: Systematic review and meta-analysis of the effects of prototypic antidepressants. Neuroscience and biobehavioral reviews, 84, 1–11. https://doi.org/10.1016/j.neubiorev.2017.11.003
- Berton, O., McClung, C. A., Dileone, R. J., Krishnan, V., Renthal, W., Russo, S. J., Graham, D., Tsankova, N. M., Bolanos, C. A., Rios, M., Monteggia, L. M., Self, D. W., & Nestler, E. J. (2006). Essential role of BDNF in the mesolimbic dopamine pathway in social defeat stress. Science (New York, N.Y.), 311(5762), 864–868. https://doi.org/10.1126/science.1120972
- Boldog, E., Bakken, T. E., Hodge, R. D., Novotny, M., Aevermann, B. D., Baka, J., Bordé, S., Close, J. L., Diez-Fuertes, F., Ding, S. L., Faragó, N., Kocsis, Á. K., Kovács, B., Maltzer, Z., McCorrison, J. M., Miller, J. A., Molnár, G., Oláh, G., Ozsvár, A., Rózsa, M., … Tamás, G. (2018). Transcriptomic and morphophysiological evidence for a specialized human cortical GABAergic cell type. Nature neuroscience, 21(9), 1185–1195. https://doi.org/10.1038/s41593-018-0205-2
- Lin, S. H., Lee, L. T., & Yang, Y. K. (2014). Serotonin and mental disorders: a concise review on molecular neuroimaging evidence. Clinical psychopharmacology and neuroscience : the official scientific journal of the Korean College of Neuropsychopharmacology, 12(3), 196–202. https://doi.org/10.9758/cpn.2014.12.3.196
- Takahashi, K., Tanabe, K., Ohnuki, M., Narita, M., Ichisaka, T., Tomoda, K., & Yamanaka, S. (2007). Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell, 131(5), 861–872. https://doi.org/10.1016/j.cell.2007.11.019
- Kim, J., Koo, B. K., & Knoblich, J. A. (2020). Human organoids: model systems for human biology and medicine. Nature reviews. Molecular cell biology, 21(10), 571–584. https://doi.org/10.1038/s41580-020-0259-3
- Lancaster, M. A., Renner, M., Martin, C. A., Wenzel, D., Bicknell, L. S., Hurles, M. E., Homfray, T., Penninger, J. M., Jackson, A. P., & Knoblich, J. A. (2013). Cerebral organoids model human brain development and microcephaly. Nature, 501(7467), 373–379. https://doi.org/10.1038/nature12517
- Mansour, A. A., Gonçalves, J. T., Bloyd, C. W., Li, H., Fernandes, S., Quang, D., Johnston, S., Parylak, S. L., Jin, X., & Gage, F. H. (2018). An in vivo model of functional and vascularized human brain organoids. Nature biotechnology, 36(5), 432–441. https://doi.org/10.1038/nbt.4127
- Jones, J. B. M. (2021, April 3). Americans Hold Record Liberal Views on Most Moral Issues. Gallup.Com. https://news.gallup.com/poll/210542/americans-hold-record-liberal-views-moral-issues.aspx?g_source=animal+testing&g_medium=search&g_campaign=tiles
- Narver, H., Hoogstraten-Miller, S., Linkenhoker, J. et al. Tributes for animals and the dedicated people entrusted with their care: a practical how-to guide. Lab Anim 46, 369–372 (2017). https://doi.org/10.1038/laban.1346
