Introduction: A General Explanation of Optogenetics
Neuroscience can only go as far as its research tools will take it. All science relies on experimentation and observation, which are difficult to accomplish when studying complex biological systems such as the brain. When it comes to experimentation, one of the major innovations in research techniques is the ability to stimulate the brain. Stimulation studies aim to stimulate sections of the brain, causing neurons to fire. They can be used to demonstrate causality, because if a behavior that is not present prior to stimulation consistently occurs following stimulation, scientists can infer a causal relationship between stimulation induced activity and that behavior. A research tool enabling stimulation studies that has received great amounts of attention in recent years is optogenetics, which is distinguished by its use of light in controlling neural activity.
Optogenetics is far from the only tool capable of brain stimulation, and to understand why it has garnered so much praise in the neuroscience community, one must first understand the strengths and limitations of other common alternatives. One of the most common and least invasive techniques is transcranial magnetic stimulation (TMS) which uses an electromagnetic field to stimulate neurons[1]. It is minimally invasive, as it does not require surgery, which also makes it less expensive than many alternatives. However, TMS is only effective on the areas of the brain near the scalp, as the magnet cannot target the brain’s inner structures without also stimulating the outer structures. In order to stimulate those inner structures, researchers use deep brain stimulation (DBS) [2]. DBS is far more invasive because the electrodes are surgically implanted in the brain, which allows the electrical signal to reach regions inaccessible to TMS. The major limitation of both techniques is that they are unspecific: the electrodes send electricity to the connected brain regions, not just the target area. This makes it hard to determine if the observed effect was caused by the target area or something around it.
Then, in 2005, a new research technique was discovered that uses no electrodes at all. Instead, it uses a gene found in algae that allows them to convert light into neural activity [3]. When the gene is inserted into neurons, researchers can stimulate specific neurons by simply shining a light on them. This technique is called optogenetics and it allows researchers both exceptional spatial resolution and cell type specificity through genetic targeting. This technique revolutionizes the way we study complex nervous systems and provides insight into some of neuroscience’s greatest questions.
The Discovery of Optogenetics
The first successful use of optogenetics was by two researchers at Stanford, Dr. Karl Deisseroth and his Ph.D student, Edward Boyden. The genes used by Boyden and Deisseroth were not a new discovery. They were discovered in the 1970s, and further studies over the years deepened the understanding of their function [4]. The genes code for proteins called opsins, which sit in the membrane of a cell. Opsins are present in both animals and microbes, but only the ones in microbes are used for optogenetics. They function as a pump or channel, allowing ions to flow down a concentration gradient and induce an electric current [5]. When exposed to light, ospins modify the behavior of a cell, which can induce or suppress action potentials when placed in neurons depending on the charge and direction of flowing ions. To do this, a form of vitamin A called retinal binds with an opsin. When the retinal is hit with light it is chemically altered, changing the shape of the opsin which opens, allowing ions to flow across the membrane.
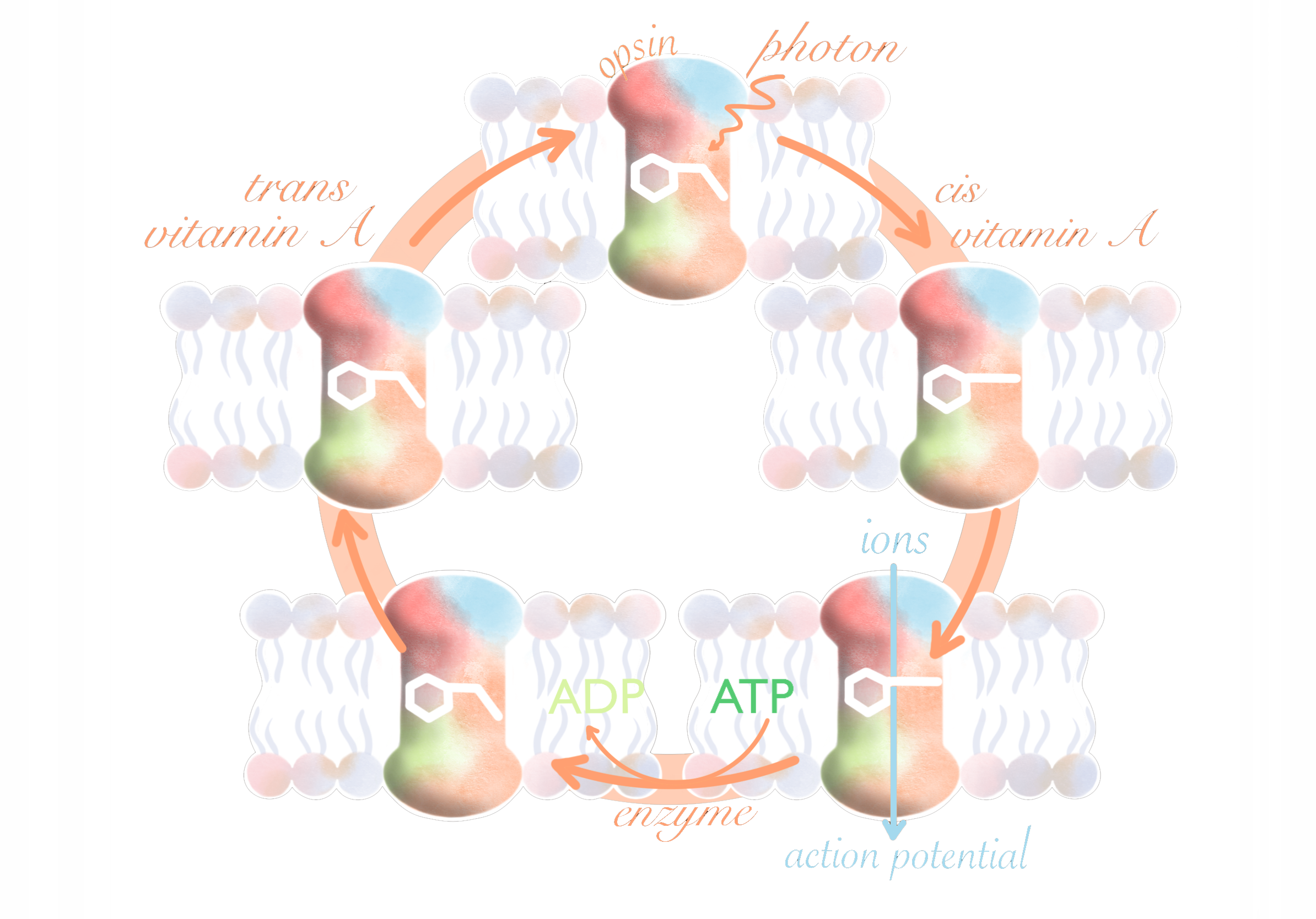
Researchers saw that if these opsins could be functional in a neuron, it would allow them to control the activity of specific neurons. Even though it had long been suspected that opsins could be used to stimulate neurons, the idea had stagnated due to the difficulty of trying to express the gene that codes for opsins in neurons. Opsins do exist in animals and also detect light; however, they don’t function as an ion channel, they trigger a slow pathway of cell responses that isn’t useful for controlling neural activity. The environment an opsin in a microbe inhabits is very different from that of an animal cell. Microbes often live in environments with very different pH levels and salinities than a neuron. Additionally, neurons have a constantly changing membrane potential that is not present in microbes. Many past attempts to express opsins from microbes in animal cells had been unsuccessful, likely because the opsins were maladapted to their new environment [3].
The first sign of hope came from Gero Miesenbock at the University of Oxford. In 2002, he published the results of an experiment that took the genes that coded for the opsins in common fruit flies and expressed them in the immature egg cells of a frog. [6] When the researchers illuminated the cells, they found that they had successfully evoked action potentials. The experiment required ten different proteins and took several seconds from illumination to action potential. However, it proved that it was possible to express the opsin genes of one organism in another which was enough to interest Edward Boyden and Karl Deisseroth.
The final ingredient was a paper by Georg Nagel at the University of Wurzburg, who discovered the opsins in green algae that allow positively charged ions to flow into the cell, bringing it closer to activation, and named it channelrhodopsin-2 (ChR2). Nagel was able to express the gene in both frog egg cells and mammalian cells, and his electrical data estimated that the activation took less than a millisecond, in contrast to the several second response time of the earlier experiment by Miesenbock [7]. This discovery was what Boyden and Deisseroth needed to start their experiments, so they contacted Nagel to ask for a clone of the ChR2 they discovered [3]. By the time they asked, Nagel had already made several improvements, including finding a fluorescent protein that could be combined with ChR2 in order to track its expression and finding a mutation that made the ChR2 less likely to stop working.
Boyden and Deisseroth worked on two different ends of the project. Deisseroth focused on trying to express the ChR2 gene in neurons and attempted to perfect the process that added the gene into cells. Boyden, on the other hand, worked on the technological aspects, programming the lights that would activate the opsins that Deisseroth was working on. According to Boyden’s account, around 1 a.m, on August 4, 2004, he placed a dish of neurons that had the gene for channelrhodopsin-2 under a microscope and started his program to pulse light onto the dish. He describes, “to my amazement, the very first neuron I patched fired precise action potentials in response to blue light” [3]. The data collected on that night and the ones that followed would form the paper in Nature Neuroscience that eventually won Boyden a Rumford Prize and launched the field of optogenetics as we know it today.
Gene Expression
So, how can we move a gene from one species to another, and how does the research organism know which cells to express the gene in? One popular technique uses engineered viruses to deliver the gene, capitalizing on the natural ability of viruses to enter a cell and insert its DNA [8]. The section of DNA that allows viruses to replicate is removed, so the virus can infect the cell, but cannot create new viruses. The viral receptor, what the virus uses to bind to host cells, can also be changed so that the virus will only target a specific type of cell, which is part of what optogenetics uses to control specific cells. Targeted expression can also be achieved if the gene is activated by a promoter that is specific to a certain cell type. Viruses are preferred because they can introduce multiple copies of the gene to one cell, which increases the gene’s expression. Their diffusion can also be controlled, as some viruses spread throughout the entire brain, while others only infect cells near the injection site.

Recent Efforts
There have been many improvements in optogenetics since its advent, as researchers work to optimize every aspect of the process. Ultrafast opsins have been discovered through targeted mutations that allow for extremely fast temporal control of neural activity at high firing rates. They also reduce the occurrence of accidentally setting off multiple action potentials which would make the data useless [9]. While the ChR2 used in Boyden’s original experiment creates an action potential, other opsins like bacteriorhodopsin and halorhodopsin can inhibit neural activity, effectively shutting down small parts of the brain. These can be used to complement other excitatory techniques, as they can inhibit certain areas to determine their function and necessity.
Another improvement in optogenetic technology is the development of opsins that respond to different colors of light, allowing for the independent control of multiple sets of neurons. For example, if you had a set of neurons that responded to red light and another set of neurons that responded to blue light, you could use the two colors of light to activate each set separately [10]. The delivery mechanism for the light has also been improved. It’s fairly easy to shine a light on a dish of cells, but the process becomes more complex in fully grown animals. Lasers are popular because they can produce a very specific wavelength of light, which is especially helpful when working with multiple types of opsins that respond to different colors. They can also be paired with optical fibers, which can help deliver light to deep brain structures. Another option is LEDs, which are smaller, cheaper, and come in a wider variety of wavelengths at the cost of providing light with a less well-defined wavelength and spatial distribution. These improvements have only scratched the surface of what can be done with optogenetics, and researchers are constantly working on ways to improve the technology even further.
Current and Potential Applications
While other stimulation methods also involve surgery, optogenetics requires gene modification, which has only recently been approved for a small number of clinical trials, so the current applications of optogenetics lie in research, although some believe that the technique could one day be useful in medicine. One way that optogenetics is used is called “cracking neural codes.” With pulses of light, we can recreate many patterns of neural activity and view the effects this induces [11]. By recording the inputs and outputs of the system, we can gain a greater understanding of the relationship between them. Another use of optogenetics is the interrogation of neural circuits. Neural circuits are a collection of neurons that are interconnected and all work together to serve one specific function. Old studies of the brain required parts of the brain to be removed, however with optogenetics, researchers can inhibit the activity of one specific element of a circuit and observe the change in function that results, to determine the necessity and use of each part of a circuit [12]. Optogenetics can also be used to study the effects and mechanisms of certain disorders and diseases that affect the brain. The precise control offered by optogenetics allows researchers to recreate the neural patterns of a disease or disorder in a test subject and either study its mechanism or test potential treatments. These models have already been created for cardiac physiology [13].
Further Research
Optogenetics has proven to be an invaluable research tool and has provided numerous insights to researchers studying the brain. However, the medical application of optogenetics is limited by the gene editing it requires [14]. Gene therapy is a very controversial topic in scientific circles with many worried that the ability to modify our genome will lead to a slippery slope, where people will start making aesthetic changes to themselves, or even their children. Scientists and doctors are also concerned about the safety of gene therapies. Unlike a surgical implant, once the gene is edited it cannot be reversed, even if it is found to be harmful. However, if optogenetics could be proven safe and effective in humans, optogenetics could radically change the medical field. Preliminary studies have shown positive results in the medical application of optogenetics. Future uses such as treating eye disorders, treating chronic pain, and serving as a deep brain stimulation alternative show that the potential of this technique has only just begun to be tapped into [15][16][17]. These new applications may be a long way off but the technique is already revolutionary. Whether in research or medicine its impact is only projected to grow.
References
[1] Chail, A., Saini, R. K., Bhat, P. S., Srivastava, K., & Chauhan, V. (2018). Transcranial magnetic stimulation: A review of its evolution and current applications. Industrial Psychiatry Journal, 27(2), 172-180. https://doi.org/10.4103/ipj.ipj_88_18.
[2] Herrington, T. M., Cheng, J. J., & Eskandar, E. N. (2016). Mechanisms of deep brain stimulation. Journal of Neuropsychology, 115(1), 19-38. https://doi.org/10.1152/jn.00281.2015.
[3] Boyden E. S. (2011). A history of optogenetics: the development of tools for controlling brain circuits with light. F1000 biology reports, 3, 11. https://doi.org/10.3410/B3-11.
[4] Yizhar, O., Fenno, L. E., Davidson, T. J., Mogri, M., & Deisseroth, K. (2011). Optogenetics in Neural Systems. Neuron, 71(1), 9-34. https://doi.org/10.1016/j.neuron.2011.06.004.
[5] Guru, A., Post, R. J., Ho, Y. Y., & Warden, M. R. (2015). Making Sense of Optogenetics. The international journal of neuropsychopharmacology, 18(11), pyv079. https://doi.org/10.1093/ijnp/pyv079.
[6] Zemelman, B. V., Lee, G. A., Ng, M., & Miesenböck, G. (2002). Selective photostimulation of genetically chARGed neurons. Neuron, 33(1), 15–22. https://doi.org/10.1016/s0896-6273(01)00574-8.
[7] Nagel, G. et al. (2003). Channelrhodopsin-2, a directly light-gated cation-selective membrane channel. Proceedings of the National Academy of Sciences of the United States of America, 100(24), 13940-5. https://doi.org/10.1073/pnas.1936192100.
[8] Bouard, D., Alazard-Dany, N., & Cosset, F-L. (2009). Viral vectors: from virology to transgene expression. British Journal of Pharmacology, 157(2), 153-165. https://dx.doi.org/10.1038%2Fbjp.2008.349.
[9] Malyshev, A., Goz, R., LoTurco, J. J., & Volgushev, M. (2015). Advantages and limitations of the use of optogenetic approach in studying fast-scale spike encoding. PloS one, 10(4), e0122286. https://doi.org/10.1371/journal.pone.0122286.
[10] Chow, B. Y., Han, X., Dobry, A. S., Qian, X., Chuong, A. S., Li, M., Henninger, M. A., Belfort, G. M., Lin, Y., Monahan, P. E., & Boyden, E. S. (2010). High-performance genetically targetable optical neural silencing by light-driven proton pumps. Nature, 463(7277), 98–102. https://doi.org/10.1038/nature08652.
[11] Mahmoudi, P., Veladi, H., & Pakdel, F. G. (2017). Optogenetics, Tools and Applications in Neurobiology. Journal of medical signals and sensors, 7(2), 71–79.
[12] Zhang, F., Gradinaru, V., Adamantidis, A. R., Durand, R., Airan, R. D., Lecea, L., & Deisseroth, K. (2010). Optogenetic interrogation of neural circuits: technology for probing mammalian brain structures. Nature Protocols, 5(3), 439-456. https://dx.doi.org/10.1038%2Fnprot.2009.226.
[13] Ferenczi, E. A., Tan, X., & Huang, C. L. (2019). Principles of Optogenetic Methods and Their Application to Cardiac Experimental Systems. Frontiers in physiology, 10, 1096. https://doi.org/10.3389/fphys.2019.01096.
[14] Gonçalves, G. A. R. & Paiva, R. M. A. (2017). Gene therapy: advances, challenges and perspectives. Einstein (Sao Paulo), 15(3), 369-375. https://dx.doi.org/10.1590%2FS1679-45082017RB4024.
[15] Gaub, B. M., Berry, M. H., Visel, M., Holt, A., Isacoff, E. Y., & Flannery, J. G. (2018). Optogenetic Retinal Gene Therapy with the Light Gated GPCR Vertebrate Rhodopsin. Methods in Molecular Biology, 1715, 177-189. https://dx.doi.org/10.1007%2F978-1-4939-7522-8_12.
[16] Lee, G. H. & Kim, S. S. (2016). Therapeutic Strategies for Neuropathic Pain: Potential Application of Pharmacosynthetics and Optogenetics. Mediators of Inflammation, 2016. https://doi.org/10.1155/2016/5808215.
[17] Sanders, T. H. & Jaeger, D. (2016). Optogenetic stimulation of cortico-subthalamic projections is sufficient to ameliorate bradykinesia in 6-ohda lesioned mice. Neurobiology of Disease, 95, 225-237. https://doi.org/10.1016/j.nbd.2016.07.021.