Introduction
Imagine you are in a car accident and break your wrist. You need surgery to repair it. Surgeries like these are relatively straightforward and can sometimes take under an hour. For such a quick surgery, it does not make sense to stay in the hospital or surgery center all night. Instead, you would benefit from going home as soon as possible. One of the major limiting factors in this is pain management. The surgical team’s goal is not only to heal you, but also to ensure that you are comfortable throughout the healing process.
One way of providing this care is by providing patients with a peripheral nerve block (PNB), a type of local anesthetic (LA). PNBs are anesthetics given to help with pain management after surgery. They are non-addictive and serve as an alternative to opioids for reducing postoperative pain at home. By allowing for patients to return home sooner, they also allow for more outpatient surgeries. In this article, we will explore how the nervous system allows you to perceive pain and how PNBs work to combat postoperative pain.
How the Brain Understands Pain
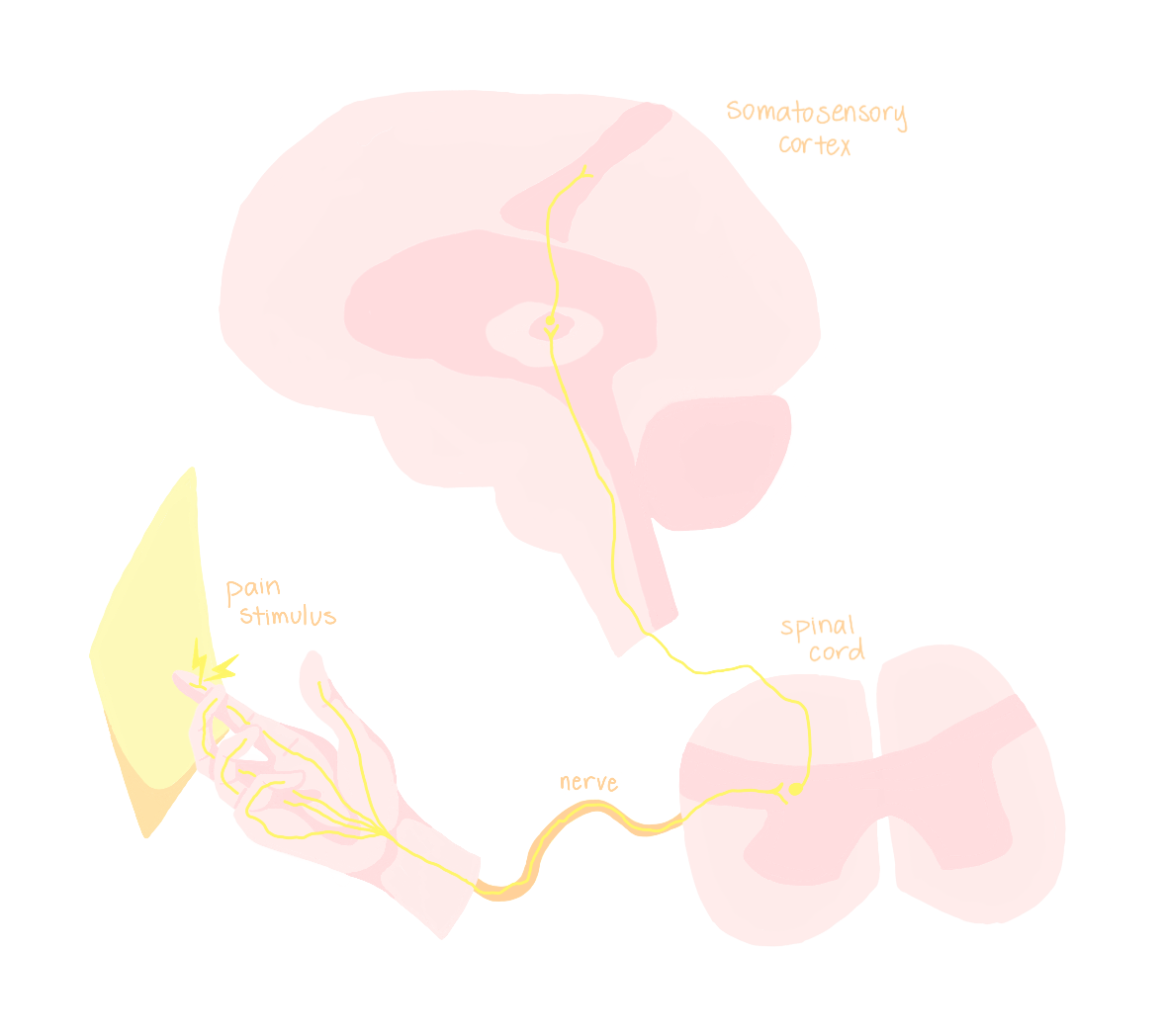
To understand pain, we must first know how painful stimuli are transmitted throughout the body. The peripheral nervous system (PNS) passes signals onto the central nervous system (CNS). The PNS is a collection of neurons and nerves all over the body that take inputs and send signals back to the CNS, which consists of the brain and spinal cord.
Imagine you get a paper cut on your finger. The first component of the pathway to sense this stimulus is a pain receptor neuron in the finger, which converts the stimulus into an electrical signal [1]. The frequency and duration of the pain receptor’s electrical firing pattern transmits information about the intensity and timing of the painful stimulus. Then, the signal travels along the neuron’s axon, which is bundled together with the axons of other sensory and motor neurons from the same area to form a peripheral nerve. The peripheral nerve terminates at the spine, at which point the signal reaches the CNS. From here, the signal is carried up the spinal cord to an area of the brain called the thalamus, which sends the signal to the somatosensory area of the cerebral cortex. The somatosensory cortex is responsible for processing sensory inputs and forwards its information to the areas of the brain controlling physical movement of the area of the body where the pain originated. This brain region, which changes based on the pain’s origin, tells your body to physically move away from the painful stimuli, such as by jerking away from the knife or piece of paper that cut you [1].
Peripheral nerve blocks are designed to reduce the pain that your brain perceives. To do this, they interrupt the signal from the painful stimulus as it travels along the nerve, before it gets to the brain [2]. Administering PNBs before surgery can therefore preemptively stop pain signals that you would otherwise “feel” after you wake up.

Context: Postoperative Pain
Pain originating from the peripheral nervous system can generally be categorized into two types: nociceptive and neuropathic [3]. In nociceptive pain, neurons fire in response to a stimulus that originates from outside the CNS. For example, nociceptive pain can arise from a painful injury, such as getting a cut, or from an inflammatory response. The body produces an inflammatory response when fighting an infection or attempting to heal damaged tissue. This damaged tissue produces pain because your baseline for pain goes down when you are injured, making everything seem more painful in a process called hypersensitization. On the other hand, with neuropathic pain the neurons themselves are damaged and send signals to the CNS in absence of a stimulus outside the nervous system [3]. Thus, nociceptive pain can be thought of as pain arising from bodily damage, while neuropathic pain is pain arising from incorrect neural signaling.
After a surgery, patients may experience postoperative pain of both categories. For example, movement near the surgical area can lead to nociceptive pain [3]. Patients might also experience breakthrough pain, which is when there is an amplification of inflammation resulting in hypersensitization of areas surrounding the injury site. For example, inflammation might be around a cut, and not just on the site of the cut. In this way, pain might be felt even in absence of an active, painful stimulus. Alternatively, patients might experience neuropathic pain from a nerve injury experienced during surgery [3].
Mitigating postoperative pain is critical in order for patients to have positive surgical experiences. In fact, postoperative pain is the most common complaint about surgeries [4, 5, 6]. It is also the most common complaint in the emergency setting, and it is often under-addressed after emergency surgery [5]. Orthopedic surgeries are especially painful and this pain is felt most acutely during the postoperative process, so PNBs are commonly used in orthopedics [7].
Postoperative pain is commonly treated with opioids. However, the ongoing opioid epidemic is encouraging a pivot toward alternative methods of pain reduction [8, 9]. This is where PNBs, which are non-addictive, are particularly useful. Two of the most common PNBs are ropivacaine and bupivacaine, as they can both provide up to 24 hours of pain relief [10]. For longer pain relief, patients can receive a PNB via a catheter, but this would mean they either need to stay in the hospital or do it at home, both of which reduce the main benefit of receiving PNBs in the first place. In most instances, this makes PNBs the better option.
How PNBs Work
Peripheral nerve blocks are thought to work by interrupting the signal transmission process along the peripheral nerve [2]. Specifically, they inhibit sodium channels, which are small proteins that lie along the axon and help enable neurons to transmit electrical signals to each other. When a peripheral nerve block is active, the transmission is blocked. As soon as the block wears off, the neurons resume firing, allowing the electrical signal to reach the synapse and communicate with the next neuron along the line. For a PNB to inhibit these channels, it must make its way between the tissue surrounding the individual nerve fibers. The extent to which it can do this is dependent on the mass and thickness of the tissue surrounding it. Because the tissue surrounding the fibers thickens as it gets further from the spine, blocks injected further from the spine take longer to be effective [2]. So, the optimal PNB is maximized in its capacity to travel through surrounding tissue and the number of sodium channels it inhibits.
Using PNBs
When an anesthesiologist injects a peripheral nerve block, their goal is to maximize precision. To achieve this, they utilize a useful form of imaging called ultrasound. While ultrasound is traditionally thought of as being used to view a fetus, it has many applications for when there is a need to visualize something under the skin, such as to make a specific cut [11]. The delivery of peripheral nerve blocks is one of these procedures. This is a relatively recent innovation that has improved the safety and effectiveness of PNBs and increased the number of surgeries that can utilize them.
Prior to using ultrasound, anesthesiologists had to rely on their training regarding the proper location of the target nerve, resulting in some guesswork for injections. Using ultrasound, anesthesiologists can visualize the nerves and other anatomical structures in the patients to which they are providing anesthesia [12]. This provides increased visibility and accuracy, allowing the anesthesiologist to ensure the drug does not spread to a location it should not be [12]. Additionally, increased visibility means blood vessels are less likely to be encountered and damaged [13]. This innovation makes previously high-risk procedures more routine and increases patient access to the positive benefits of PNBs.
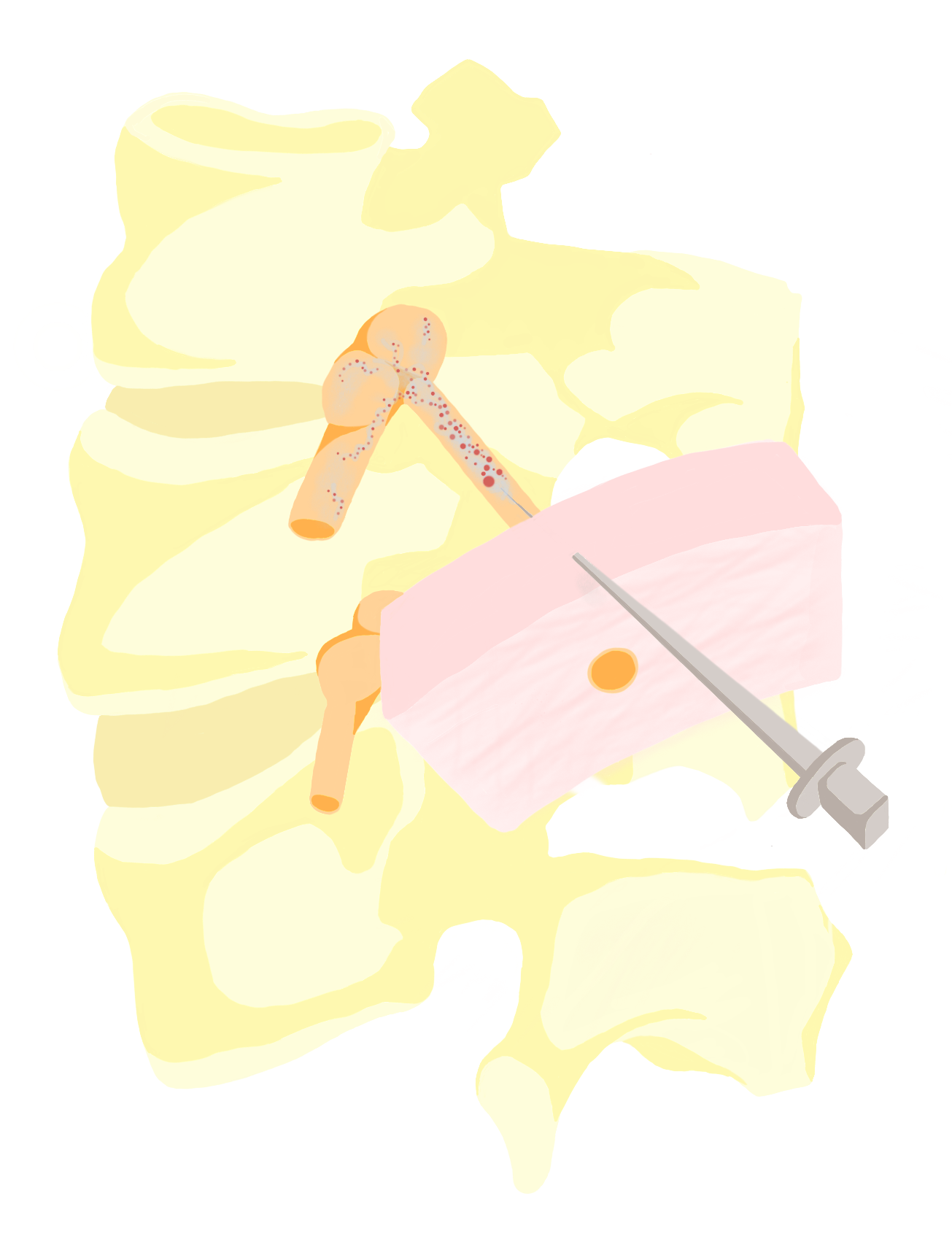
Moreover, increased accuracy from ultrasound guidance means that an anesthesiologist does not have to provide higher dosages in the hopes that they hit the specific target nerve [14]. A larger percentage of the dose goes to the intended area, making the anesthetic more effective for any given dosage. This is important since higher dosages increase the risk of unintended neurotoxicity, or adverse effects that can occur when foreign substances are introduced to the nervous system. Additionally, more precise targeting also reduces the chance a second block would need to be administered [14].
Adjuncts
Peripheral nerve blocks can be given in conjunction with other drugs, called adjuncts, to enhance or modify their effects. The major properties that adjuncts change are the time it takes for a PNB to be effective and the length of time it provides pain relief after surgery [2]. One way to achieve these long-lasting effects is to inject small storage pouches containing a local anesthetic near the area of surgery. Recall that PNBs are a type of local anesthetic. Additionally, the pouch contains the adjunct drug. After the surgery, these pouches break down over time, releasing the PNB adjunct drug and providing a mechanism for delayed release [2].
One example of a long-lasting adjunct is dexamethasone, an anti-inflammatory steroid that targets one type of fiber in the peripheral nerve [2, 10]. Researchers studied the benefits of dexamethasone and LAs for people who had undergone collarbone surgery to study how well dexamethasone works on its own versus when paired with a PNB. To do this, they injected participants’ nerves near the collarbone with either ropivacaine, a PNB, or a combination of ropivacaine and dexamethasone [15]. The researchers then measured the amount of time between when the initial sensory block began and the time when the patient first requested a follow up painkiller. When patients were given both drugs, this time period was over 19.5 hours, but less then 9.5 hours when only ropivacaine was given [15]. Not only does this extra time ease a patient’s recovery, but it also means the surgeon can be less worried about completing the surgery in the initially planned length of time because the anesthetic lasts longer.
Dexmedetomidine is another adjunct that has similar time extension properties, but its other benefits allow it to be used in different types of surgeries. Specifically, it is most effective in extending anesthesia by a few hours in surgeries involving the upper extremities [10]. It can also be administered orally, which makes it particularly useful for pediatric surgeries. An additional benefit to dexmedetomidine over other adjuncts is its lack of known neurotoxic side effects [16].
Finally, ketamine can also serve as an adjunct. The pain management properties of ketamine were first found in 1965 [17]. Among its many effects, it is involved in dampening pain signals. Specifically, ketamine works by blocking NMDA receptors on neurons in the pain signaling pathway, preventing them from being excited. This dissociates the experience of pain from the painful peripheral stimuli because the signal never reaches the central nervous system. One benefit to using ketamine as a PNB is that it targets the same pathways that opioids do, while not having the same tolerance building [18]. However, its addictive properties are still being explored.
Stakes and Future Research
As it relates to opioid usage, there are two theories for why PNBs could decrease risk for persistent opioid consumption. The first theory is known as preventative analgesia [9]. Under this theory, if an anesthetic is administered before surgery, it can help block transmission of pain during the operation procedure [19]. This means that pain pathways are blocked before surgery. This is beneficial because it means that once the patient wakes up, they do not feel pain. If the anesthesiologist waits to administer it until after the end of surgery, it will not be fully effective and a patient will wake up in pain. Through this process, PNBs have the potential to reduce nociceptive pain since the pathways that would otherwise be amplified after surgery are numbed before the fact [19]. The second theory is based on experimental evidence that nerve blockers can be used to treat acute, postoperative pain [9]. Such pain is a risk factor for persistent opioid consumption [3].
By reducing nociceptive pain, PNBs enable patients to return home faster and earlier. Thus, surgeries that have historically been inpatient can be done as outpatient procedures. This is important because outpatient procedures are associated with lower recovery room times and lower hospital readmission rates, and constitute about half of all surgeries in the United States [20]. Despite these benefits, PNBs are underutilized, especially in the emergency setting and in under-resourced areas due to a lack of anaesthesiologist training [21, 22, 23].
While PNBs are now an established part of anesthesiology, there are still some unanswered questions and limitations in their effectiveness. Some fibers show resistance to PNBs, but no one is exactly sure why, warranting future research on this complication. Another ongoing area of research concerns the creation, benefits, and drawbacks of a “cocktail,” or combination of adjuncts, that are administered together [10]. Furthermore, PNBs only interrupt the initial wave of pain signals that lead to feeling sharp, instantaneous pain [2]. PNBs are not effective in reducing the secondary, long, dull pain sensations you feel after the immediate, painful stimulus. These signals travel up a separate set of fibers that are slower and resistant to local anesthetics like PNBs [2]. Understanding why this is the case, and finding answers to these questions will allow for the development of more precise, safer, and effective anesthetics, making the postoperative recovery process smoother for patients.
References
[1] Kandel, E. R., Koester, J., Mack, S., & Siegelbaum, S. (2021). Principles of neural science (Sixth edition.). McGraw Hill.
[2] Vadhanan, P., Tripaty, D. K., & Adinarayanan, S. (2015). Physiological and pharmacologic aspects of peripheral nerve blocks. Journal of Anaesthesiology, Clinical Pharmacology, 31(3), 384–393. https://doi.org/10.4103/0970-9185.161679
[3] Kehlet, H., Jensen, T. S., & Woolf, C. J. (2006). Persistent postsurgical pain: Risk factors and prevention. The Lancet, 367(9522), 1618–1625. https://doi.org/10.1016/S0140-6736(06)68700-X
[4] Wu, C. L., Berenholtz, S. M., Pronovost, P. J., & Fleisher, L. A. (2002). Systematic Review and Analysis of Postdischarge Symptoms after Outpatient Surgery. Anesthesiology, 96(4), 994–1003. https://doi.org/10.1097/00000542-200204000-00030
[5] Todd, K. H., Ducharme, J., Choiniere, M., Crandall, C. S., Fosnocht, D. E., Homel, P., & Tanabe, P. (2007). Pain in the Emergency Department: Results of the Pain and Emergency Medicine Initiative (PEMI) Multicenter Study. The Journal of Pain, 8(6), 460–466. https://doi.org/10.1016/j.jpain.2006.12.005
[6] Nischal, N., Arulraja, E., & Shaheen, S. P. (2020). Pain Management for Orthopedic Injuries. Emergency Medicine Clinics of North America, 38(1), 223–241. https://doi.org/10.1016/j.emc.2019.09.013
[7] Gerbershagen, H. J., Pogatzki-Zahn, E., Aduckathil, S., Peelen, L. M., Kappen, T. H., van Wijck, A. J. M., Kalkman, C. J., & Meissner, W. (2014). Procedure-specific Risk Factor Analysis for the Development of Severe Postoperative Pain. Anesthesiology, 120(5), 1237–1245. https://doi.org/10.1097/ALN.0000000000000108
[8] Kolodny, A., Courtwright, D. T., Hwang, C. S., Kreiner, P., Eadie, J. L., Clark, T. W., & Alexander, G. C. (2015). The Prescription Opioid and Heroin Crisis: A Public Health Approach to an Epidemic of Addiction. Annual Review of Public Health, 36(1), 559–574. https://doi.org/10.1146/annurev-publhealth-031914-122957
[9] Hah, J. M., Bateman, B. T., Ratliff, J., Curtin, C., & Sun, E. (2017). Chronic Opioid Use After Surgery: Implications for Perioperative Management in the Face of the Opioid Epidemic. Anesthesia and Analgesia, 125(5), 1733–1740. https://doi.org/10.1213/ANE.0000000000002458
[10] Kuo, B., & Ortiz, J. (2020). Adjuncts to Local Anesthetics for Peripheral Nerve Blocks: A Review of Current Literature. Topics in Pain Management, 35(12), 1. https://doi.org/10.1097/01.TPM.0000681628.38413.fc
[11] Mercaldi, C. J., & Lanes, S. F. (2013). Ultrasound guidance decreases complications and improves the cost of care among patients undergoing thoracentesis and paracentesis. Chest, 143(2), 532–538. https://doi.org/10.1378/chest.12-0447
[12] Dufeu, N., Marchand-Maillet, F., Atchabahian, A., Robert, N., Ait Yahia, Y., Milan, D., Robert, C., Coroir, M., & Beaussier, M. (2014). Efficacy and Safety of Ultrasound-Guided Distal Blocks for Analgesia Without Motor Blockade After Ambulatory Hand Surgery. The Journal of Hand Surgery, 39(4), 737–743. https://doi.org/10.1016/j.jhsa.2014.01.011
[13] Abrahams, M. S., Aziz, M. F., Fu, R. F., & Horn, J.-L. (2009). Ultrasound guidance compared with electrical neurostimulation for peripheral nerve block: A systematic review and meta-analysis of randomized controlled trials. British Journal of Anaesthesia, 102(3), 408–417. https://doi.org/10.1093/bja/aen384
[14] Melnyk, V., Ibinson, J. W., Kentor, M. L., & Orebaugh, S. L. (2018). Updated Retrospective Single-Center Comparative Analysis of Peripheral Nerve Block Complications Using Landmark Peripheral Nerve Stimulation Versus Ultrasound Guidance as a Primary Means of Nerve Localization. Journal of Ultrasound in Medicine, 37(11), 2477–2488. https://doi.org/10.1002/jum.14603
[15] Kumar, S., Palaria, U., Sinha, A. K., Punera, D. C., & Pandey, V. (2014). Comparative evaluation of ropivacaine and ropivacaine with dexamethasone in supraclavicular brachial plexus block for postoperative analgesia. Anesthesia, Essays and Researches, 8(2), 202–208. https://doi.org/10.4103/0259-1162.134506
[16] Tüfek, A., Kaya, S., Tokgöz, O., Fırat, U., Evliyaoğlu, O., Çelik, F., & Karaman, H. (2013). The protective effect of dexmedetomidine on bupivacaine-induced sciatic nerve inflammation is mediated by mast cells. Clinical and Investigative Medicine, 36(2), E95–E102. https://doi.org/10.25011/cim.v36i2.19572
[17] Domino, E. F., Chodoff, P., & Corssen, G. (1965). Pharmacologic effects of CI-581, a new dissociative anesthetic, in man. Clinical Pharmacology & Therapeutics, 6(3), 279–291. https://doi.org/10.1002/cpt196563279
[18] Iacobucci, G. J., Visnjevac, O., Pourafkari, L., & Nader, N. D. (2017). Ketamine: An Update on Cellular and21 Subcellular Mechanisms with Implications for Clinical Practice. Pain Physician, 20(2), E285-E301. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/28158165
[19] Dahl, J. B., & Møiniche, S. (2005). Pre-emptive analgesia. British Medical Bulletin, 71(1), 13–27. https://doi.org/10.1093/bmb/ldh030
[20] Gabriel, R. A., & Ilfeld, B. M. (2018). Use of Regional Anesthesia for Outpatient Surgery Within the United States: A Prevalence Study Using a Nationwide Database. Anesthesia and Analgesia, 126(6), 2078–2084. https://doi.org/10.1213/ane.0000000000002503
[21] Pennington, N., Gadd, R. J., Green, N., & Loughenbury, P. R. (2012). A national survey of acute hospitals in England on their current practice in the use of femoral nerve blocks when splinting femoral fractures. Injury, 43(6), 843–845. https://doi.org/10.1016/j.injury.2011.10.003
[22] Ritcey, B., Pageau, P., Woo, M. Y., & Perry, J. J. (2016). Regional Nerve Blocks For Hip and Femoral Neck Fractures in the Emergency Department: A Systematic Review. CJEM, 18(1), 37–47. https://doi.org/10.1017/cem.2015.75
[23] Zewdie, A., Debebe, F., Azazh, A., Salmon, M., & Salmon, C. (2017). A survey of emergency medicine and orthopaedic physicians’ knowledge, attitude, and practice towards the use of peripheral nerve blocks. African Journal of Emergency Medicine, 7(2), 79–83. https://doi.org/10.1016/j.afjem.2017.04.003