An Introduction to Sensorineural Hearing Loss
For a large proportion of the population, hearing is a passive process easily taken for granted despite the complex mechanisms required for its function. Due to the intricate structures responsible for hearing, there are myriad ways that hearing loss can occur. The predominant type of hearing loss is Sensorineural Hearing Loss (SNHL), which is caused by either an infection, illness, or acoustic trauma of the inner ear [1]. Within the umbrella of SNHL is Noise-Induced Hearing Loss (NIHL), which is caused by acoustic trauma due to frequent loud noise exposure that damages sensitive structures of the inner ear [2]. Sounds that cause acoustic trauma are usually measured to be at or above 85 dBA, which is synonymous with a concert or noisy football crowd. The louder the sound, the less exposure time is required for NIHL to occur [2]. When the ear undergoes acoustic trauma, tiny cells called hair cells are the first to die, which then leads to hearing loss via a cascade of events. The fact that hair cells can die is particularly problematic because hair cell numbers in the human ear are fixed at roughly 15,000 during embryonic development, meaning they cannot grow back naturally later in life [3]. Therefore, understanding noise exposure and intensity is crucial to understanding how to avoid hearing loss.
Protective measures such as noise-canceling headphones or earplugs can help mitigate the frequent loud noise exposure for people who work in professions such as construction, factory work, or airport marshaling. However, acoustic trauma is not exclusive to these environments and anyone is susceptible to loud noise exposure, intentional or not. SNHL affects 5% of the world's population [3], yet there are few therapies beyond cochlear implants and hearing aids to alleviate the impacts of hearing loss due to the complexity of the inner ear and a general lack of understanding of the neuroscience behind hearing loss. New and upcoming therapies in regeneration of hair cells have the potential to restore hearing function.
How Does Hearing Work?
Hearing involves a complex set of mechanisms for converting sound waves through the external ear to the brain. When a sound wave reaches your head, it is funneled through your outer ear into your ear canal, where it distorts a structure called the tympanic membrane. Small bones leverage this movement to a structure called the oval window, which initiates movement of a fluid called perilymph. Similar to the wave machines at theme parks, the perilymph becomes a wave that moves through two structures called the scala vestibuli and the scala tympani, which triggers fluid movement in the cochlea, a structure closely resembling a snail shell. Instead of being filled with snail flesh, the cochlea is filled with a fluid called endolymph, and is home to a structure called the organ of Corti, which is a structure responsible for producing nerve impulses via tiny cells called hair cells [4], which are appropriately named since they have tiny hairs (stereocilia) protruding from their top side. The stereocilia are displaced laterally by the movement of an overlying structure called the tectorial membrane, which moves in response to the movement of endolymph through the cochlear duct. When the hair cells become excited by this movement (as anyone would be to ride the wave machine), they release a neurotransmitter that activates bipolar ganglion cells. These cells are a group of neuronal cell bodies that receive input from the hair cells of the organ of Corti. In response to the activation of the auditory spiral ganglion, a signal is then sent to cranial nerve VIII, which eventually enters the auditory region of the brain stem. What started as a simple sound wave can be turned into something comprehensible by the brain [4].
What Happens to your Brain When You Lose Hearing Function?
Imagine a simple circuit in which several bulbs are connected to a battery. All bulbs will have roughly equal brightness, but when one bulb and its wires are removed, the remaining bulbs shine brighter. Now imagine that the wires are neurons and the bulbs are primary senses. While a simplified analogy, this is the basic premise of how the brain compensates for a loss of a sense, such as hearing function. The brain will rearrange and redistribute resources to account for changes in the structure and functions, as a means of adapting to experiences, thus allowing the rest of the bulbs to still shine.
Hair cell death is usually what causes this “hearing light bulb” to be removed, which is characterized by permanent loss of the cells’ excitatory functions, therefore resulting in the severing of the “wires” or neurons necessary for relaying the signal to the brain. With a lack of continuous input, the structural and functional integrity of the nerves necessary for the propagation of these signals depletes over time, leading to nerve cell death [5]. When these sensory signals no longer have a path to the brain, then the regions of your brain that were once tasked with analyzing auditory signals no longer serve their purpose. Generally when this occurs, other areas of your brain in charge of your other senses tend to take over these loss-of-function regions, a process called neuroplasticity [6].
Neuroplasticity is found in adults with congenital hearing loss, as well as age or environmentally-related hearing loss (also known as post-lingual hearing loss, which falls under the category of SNHL). Post-lingual hearing loss is another term to describe people who become hearing impaired later in life, so they tend to already have the neural pathways associated with hearing before they gradually lose auditory function. Interestingly, auditory regions of the brain in people with acquired hearing loss were shown to be activated when they were actually processing visual motion and complex visual patterns, which is not seen for people without hearing loss [6]. Though this is evidence of neuroplasticity, one particular uncertainty regarding post-lingual hearing loss is that the onset of hearing loss tends to be a progression over many years. As a result of this, the degree of sensory deprivation required to induce reorganization of the brain is still unclear, however it does not diminish the evidence that neuroplasticity still plays a crucial role in sensory processing within later onset hearing loss.
In a study on the impact of later onset hearing loss on the auditory and visual systems of the brain, Sharma and colleagues found hearing loss, no matter its severity, results in diminished function of areas of the brain associated with listening, which likely leads to an increase in listening effort and cognitive workload [7]. The frontal lobe, while normally associated with thought and decision-making, assumes a greater role in auditory comprehension after a certain degree of hearing loss occurs. As a result, neuroplasticity via other functions, such as increased touch perception, may also happen. As seen in older adults, there is a strong association between hearing loss and cognitive decline, as evidenced in the pattern of high Alzheimer's and dementia incidence in people with hearing loss, which could be due to the reallocation of function minimizing the amount of energy available for other cognitive tasks [7]. Therefore, therapies to limit the severity of hearing loss in people with SNHL could provide beneficial outcomes in cognitive function and ability.
What are Current Hearing Loss Therapies and Their Potential Shortcomings?
The only hearing loss therapies on the market are hearing aids and cochlear implants (CIs), which is why research for regeneration of cochlear tissue for hearing restoration is becoming such a hot topic in clinical research. Currently, hearing aids help patients who have lost more than 30-50% of their hair cells by magnifying the sound vibrations entering the ear [9]. The fewer hair cells there are, the larger the hearing aid amplification needed to elicit function, but this is limited in that there still needs to be some hair cells for hearing aids to have a baseline level of function. When a hearing aid amplifies a sound, the existing hair cells detect these vibrations and convert them into neural signals that can then be processed by the auditory regions of the brain [10].
Though hearing aids can be assistive for people with SNHL, individual usage is relatively low. While several types of hearing aids exist, there are large variations in technology and overall fit. They are also generally not covered by health insurance, leaving the individual to pay around $1,000 to $6,000 per single aid- something that is not economically feasible for most [11]. Hearing aids are also not as effective in noisy social settings because of a phenomenon called loudness recruitment, which is characterized by an abnormally rapid increase in brain activity, which leads to the perception of louder noises after long-term use of a hearing aid. Loudness recruitment distorts fluctuations in sound level that are critical for the perception of speech; therefore, when combined with hearing loss, there is only a small range of levels in which sound is both understandable and comfortable [11]. Not only are there differences in sound level perception, there are also changes in brain structure following hearing loss that may also impair the perception of speech in other ways. For example, the brain can undergo neuroplastic changes to account for these alterations in frequency signaling from the inner ear, which further distorts the perception of sound [11]. Therefore, to restore normal auditory perception, hearing aids must not only provide amplification but also transform incoming sound to correct distortions in neural processing that result from these inevitable brain modifications. Even in people with mild to moderate hearing impairment, identifying the transformation required for creating the desired neural activity is extremely difficult. When this is paired with other factors, including lack of insurance coverage and the resulting high costs of the aid itself in addition to required consultations, fittings, and readjustments, most people tend to opt out of it altogether [11].
Another therapy for hearing loss is cochlear implants (CIs). Unlike hearing aids that work by amplifying sound, CIs bypass the damaged inner ear to deliver sounds directly to the auditory nerve [12]. Cochlear implants use a sound processor that fits behind the ear, on the outside of the head, and captures sound signals and sends them to a receiver that is implanted under the skin behind the ear. The receiver then sends the signals to electrodes implanted in the cochlea, which subsequently stimulate the auditory nerve, sending the signal to the brain. The brain interprets these signals as sounds, however, it does not sound exactly like natural hearing and usually takes users multiple months to make considerable progress in understanding speech [12].
CIs can improve hearing in people with severe hearing loss (from birth or SNHL) who are no longer helped by the use of hearing aids. CIs may be placed in one or both ears, with most adults often having one CI and one hearing aid at first, and then transitioning to two if hearing loss advances in the ear with the hearing aid [12]. For infants or toddlers in their crucial developmental period, however, CIs are usually placed in both ears at the same time to assist in learning and processing language [12]. One benefit of CIs is that they are usually at least partially covered by insurance, which can lower accessibility barriers compared to hearing aids [12]. Like hearing aids, CIs are still highly variable from person to person in their outcomes, and it is also very difficult to predict what these outcomes would be for pre implant patients [13]. Therefore, more research is required to understand how exactly neuroplasticity affects brain reorganization following hearing loss, as well as how these changes can be accounted for to ensure that all patients who are candidates for CIs can obtain maximum benefits. To account for the limitations of CIs and hearing aids, novel interventions involving stem cells could provide additional options and flexibility for people with SNHL.
Enter: Regeneration
Novel therapies for hearing loss include the exciting prospects of stem cells and their regenerative abilities. For someone experiencing SNHL, the number of hair cells decreases significantly to the point where there are not enough cells for adequate propagation of a hearing signal to the brain. However, since the recent discovery of inner ear regeneration in non-mammalian species, attention has been placed on activating a similar response in humans to restore hearing function [3,14]. Exciting new therapies in early-stage clinical trials suggest that hair cell regeneration to elicit restorative responses to hearing could potentially alleviate difficulties individuals may have while living with hearing loss [3]. Regeneration is achieved through stem cells, which act as the precursors to differentiated cells with specific functions crucial for body repair systems. Using stem cells specifically programmed to differentiate into hair cells can potentially help recover the loss of these cells within a patient and ultimately restore hearing function. Furthermore, the transplantation of new hair cells into a patient for restorative hearing function could be a future possibility, though the effectiveness of transplantation is still largely unknown [15].
One specific method for restoring hearing function involves using preexisting cells within the cochlea. When given certain enzymes necessary for cell division and multiplication, cochlear tissue cells subsequently give rise to progenitor cells. Progenitor cells can be understood as intermediate stem cells, able to differentiate into specialized cell types of a certain parent tissue or organ. Cells usually divide many times for tissue repair, and once these cells have increased in number, they can then be triggered via different signaling molecules to undergo differentiation into specific cells that serve useful functions, such as hair cells.
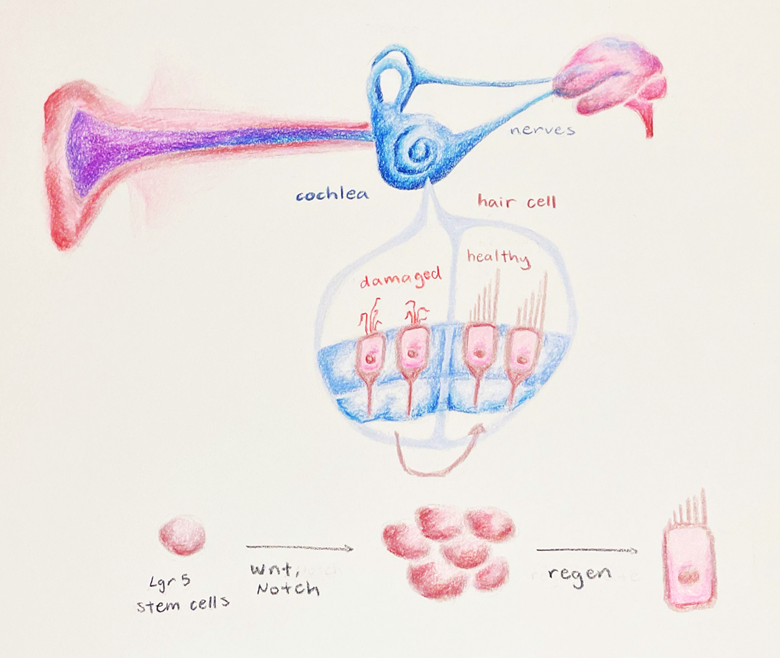
A Current Avenue of Research: Lgr5 Cells
Progenitor cell differentiation is a current target for understanding how to increase regeneration of hair cells in the adult human cochlea, but it is a complex mechanism with many moving parts. Two parts that are particularly crucial in the process of cell division and differentiation are the Wnt (pronounced “Wint'') and Notch signaling pathways. Wnt signaling is a series of molecular events initiated by the binding of proteins called Wnt to a specific receptor on the target cell. Binding causes a series of chemical changes within the cell, called a signal cascade, which ultimately changes the expression of specific genes related to cell differentiation and proliferation. The Wnt pathway is required for the spontaneous regeneration of hair cells in the neonatal cochlea, and increasing its activity will cause an increased rate of cell division [16,17]. The ability of progenitor cells to further differentiate into hair cells can be improved by chemical or genetic changes to the Notch pathway, specifically changes that reduce the function of Notch signaling. Chemical changes of this type generally rely on an enzyme called gamma secretase, which prevents the release of a small protein that regulates how genes are transformed into readable codes to build proteins [18].
Though this method appears to be well-established, research has thus far only been conducted on newborn rodents. Researchers are still trying to understand what happens during gestational maturation that results in a loss of flexibility for the progenitor cell fate, as well as which molecular targets could be modified to increase the time window that tissue repair can occur [3]. The answer to this could lie in specific cells called Lgr5 cells, which are cells that contain large amounts of the Lgr5 protein and are associated with regenerative capabilities in the cochlea. Lgr5 cells were originally found in intestinal cells, but within the past ten years, researchers have discovered that they are also found in cochlear-supporting cells that surround hair cells [19]. Stem cells highly enriched in Lgr5 are most likely to differentiate into hair cells. Similar to progenitor cells, supporting cells with Lgr5 either divide into more supporting cells or into functional hair cells. On the other hand, cells without Lgr5 do not give rise to functional hair cells [16].
Though this is an exciting discovery, there are still some roadblocks in regeneration capacity. Due to the limitations of the time window in which regeneration can occur, it is difficult to determine whether adequate hair cell regeneration can occur in humans to properly restore hearing function Another challenge is ensuring that there are enough Lgr5 cells in the cochlea prior to inducing differentiation into hair cells. Studies on progenitor cells within the inner ear have been limited in scope due to the naturally occurring small number of Lgr5 cells, which only comprise a subset of a few stem cells in the cochlear tissue [3]. In an attempt to remove these barriers, McLean’s team applied a cocktail of drugs, which allowed them to figure out how to expand populations of Lgr5 cells [3]. It has also been shown that this same mixture of drugs drives the generation of hair cells and supporting cells in both healthy and damaged mouse neonatal organs of Corti.
To further understand the scope of this study, it is important to have an idea of the methods used to carry out such a complicated experiment. McLean’s team used mice with Lgr5 to test which methods are most effective in expanding single Lgr5 supporting cells isolated from the neonatal cochlea. Researchers then added several inhibitors and growth factors, such as Wnt to significantly increase the overall number of Lgr5 cells in culture. Without Wnt stimulation, labeled Lgr5 expression decreased due to a decline in regeneration. However, this effect was found to be reversible by re-stimulating the Wnt pathway, allowing these cells to live for extended periods of time outside of the body. Further research was conducted to identify other factors that could result in prolonged life expectancy of these cells in culture. By introducing slight alterations to growth factors as well as vitamins for necessary cellular processes, Lgr5 cell numbers consistently increased. From this discovery, McLean and colleagues examined the relative importance of each growth factor used by removing each growth factor to determine its effect on cell proliferation. From these studies, they were successfully able to isolate which growth factors were most important to Lgr5 expression. However, getting these cells to then differentiate into functional hair cells is the next crucial step to elicit restorative hearing function.
The process of increasing hair cell numbers was tested on the same Lgr5 mice with the same inhibitor previously used to differentiate inner ear progenitor cells. Following 10 days of differentiation, the expression of Lgr5 was diminished, suggesting that these cells had successfully differentiated into hair cells [3]. To understand how this method may be used in adult cochlear tissue, researchers used the same drugs and growth factors to generate clonal colonies positive for Lgr5 in human inner ear tissue. While this sample formed colonies after 12 days, their expansion was not as robust as the human neonatal cells, suggesting that additional treatments may be required for a much larger growth of Lgr5 cells specifically in adult cochlear tissues. Another limitation that will require much more research to understand is how the expansion of Lgr5 cells can be done directly in the patient’s cochlea. Current research is only expanding Lgr5 cells within the laboratory, but transplantation of these cells into a patient would be difficult to accomplish due to the delicate structure of the cochlea. These factors show that there is still a lot of work to be done to reach full regenerative capacity. Still, this work suggests that a small-molecule approach, such as introducing inhibitors and growth factors to activate Wnt and Notch, could be a viable therapeutic route to restore hair cells in adults [3]. McLean’s identification of the drug cocktail is significant in that further studies can be done with slight alterations to understand which mixture is most effective in regenerating hair cells within adult cochlear tissue, and how these can be administered effectively to the patient.
Final Words
Hearing can be thought of as a multidisciplinary entity that not only encapsulates complex mechanisms leading to intricate neural pathways, but also the social and cultural identities that allow it to blossom in both its presence and absence. Culture, in a broad sense, tends to be associated with nationality, yet there is also a culture of Deafness that is important to the identities of a large portion of the population. Therefore, hearing loss cannot be considered something that inherently needs to be fixed, but there should be options for those who may want to improve their hearing abilities. Hearing aids and cochlear implants do provide considerable benefits when they work in harmony with the subject’s experience, but they tend to have substantial limitations in part due to their high costs and low rates of insurance coverage. Their mechanistic constraints also require considerable fine-tuning to match each unique ear and can cost significant money and time.
Instead of fine-tuning a device to fit the ear, researchers are attempting to fine-tune the ear itself. Regenerative therapies such as the current research with Lgr5 positive cells could be a huge promise in restoring structural and functional integrity of the inner ear tissue through the regrowth of hair cells. The use of Lgr5-positive cells in regeneration of hair cells shows major promise in neonatal cochlear tissue, with some effect in the adult cochlea. Understanding the exact mixture of drugs to allow for the adequate regeneration of adult cochlear tissue is still in the works, but it has been suggested that a perfect blend of inhibitors and growth factors to activate the ubiquitous Wnt and Notch pathways can be the answer to these therapeutic routes.
On top of the research behind molecular targeting of crucial developmental pathways in the inner ear tissue, there is also considerable effort being directed toward understanding the driving force behind neuroplasticity. Hearing function is deeply connected to the sensorineural pathways of the brain, which can change substantially when loss of hearing function occurs in patients with SNHL/NIHL. Simply restoring hair cells may not mean that the neural connections between hair cells and the brain are established, so further research is required to ensure that hair cell regeneration directly corresponds to adequate hearing function. Nonetheless, if Lgr5 positive progenitor cells can be effectively grown and maintained long enough to differentiate into hair cells in the human adult cochlea, as well as effectively integrate with the brain, then the options for people seeking therapies for hearing loss will broaden significantly and provide appreciable benefit.
References
- Tanna, R. J., Lin, J. W., & De Jesus, O. (2022). Sensorineural hearing loss. National Library of Medicine. https://www.ncbi.nlm.nih.gov/books/NBK565860/
- U.S. Department of Health and Human Services. Noise-induced hearing loss. National Institute of Deafness and Other Communication Disorders. Retrieved April 19, 2022, from https://www.nidcd.nih.gov/health/noise-induced-hearing-loss
- McLean, W. J., Yin, X., Lu, L., Lenz, D. R., McLean, D., Langer, R., Karp, J. M., & Edge, A. S. B. (2017). Clonal expansion of LGR5-positive cells from mammalian cochlea and high-purity generation of sensory hair cells. Cell Reports, 18(8), 1917–1929. https://doi.org/10.1016/j.celrep.2017.01.066
- Felten, D. L., Maida, M. S., & Netter, F. H. (2019). Netter's Neuroscience Coloring Book (1st ed.). Elsevier.
- Ryugo, D. (2014). Auditory neuroplasticity, hearing loss and cochlear implants. Cell and Tissue Research, 361(1), 251–269. https://doi.org/10.1007/s00441-014-2004-8
- Campbell, J., & Sharma, A. (2014). Cross-modal reorganization in adults with early stage hearing loss. PLoS ONE, 9(2). https://doi.org/10.1371/journal.pone.0090594
- Sharma, A., & Glick, H. (2016). Cross-modal reorganization in clinical populations with hearing loss. Brain Sciences, 6(1), 4. https://doi.org/10.3390/brainsci6010004
- Community and culture. National Association of the Deaf. (2022). https://www.nad.org/resources/american-sign-language/community-and-culture-frequently-asked-questions/
- Centers for Disease Control and Prevention. (2020, November 24). How does loud noise cause hearing loss? Centers for Disease Control and Prevention. https://www.cdc.gov/nceh/hearing_loss/how_does_loud_noise_cause_hearing_loss.html
- U.S. Department of Health and Human Services. Hearing aids. National Institute of Deafness and Other Communication Disorders. https://www.nidcd.nih.gov/health/hearing-aids#hearingaid_02
- Lesica, N. A. (2018). Why do hearing aids fail to restore normal auditory perception? Trends in Neurosciences, 41(4), 174–185. https://doi.org/10.1016/j.tins.2018.01.008
- Mayo Foundation for Medical Education and Research. (2022, May 10). Cochlear implants. Mayo Clinic. https://www.mayoclinic.org/tests-procedures/cochlear-implants/about/pac-20385021
- Pisoni, D. B., Kronenberger, W. G., Harris, M. S., & Moberly, A. C. (2017). Three challenges for future research on cochlear implants. World Journal of Otorhinolaryngology - Head and Neck Surgery, 3(4), 240–254. https://doi.org/10.1016/j.wjorl.2017.12.010
- Schilder, A. G. M., Su, M. P., Mandavia, R., Anderson, C. R., Landry, E., Ferdous, T., & Blackshaw, H. (2019). Early phase trials of novel Hearing therapeutics: Avenues and opportunities. Hearing Research, 380, 175–186. https://doi.org/10.1016/j.heares.2019.07.003
- Roccio, M., Senn, P., & Heller, S. (2020). Novel insights into inner ear development and regeneration for targeted hearing loss therapies. Hearing Research, 397, 107859. https://doi.org/10.1016/j.heares.2019.107859
- Bramhall, N. F., Shi, F., Arnold, K., Hochedlinger, K., & Edge, A. S. B. (2014). LGR5-positive supporting cells generate new hair cells in the postnatal cochlea. Stem Cell Reports, 2(3), 311–322. https://doi.org/10.1016/j.stemcr.2014.01.008
- Hu, L., Lu, J., Chiang, H., Wu, H., Edge, A. S., & Shi, F. (2016). Diphtheria toxin-induced cell death triggers wnt-dependent hair cell regeneration in neonatal mice. The Journal of Neuroscience, 36(36), 9479–9489. https://doi.org/10.1523/jneurosci.2447-15.2016
- Maass, J. C., Gu, R., Basch, M. L., Waldhaus, J., Lopez, E. M., Xia, A., Oghalai, J. S., Heller, S., & Groves, A. K. (2015). Changes in the regulation of the notch signaling pathway are temporally correlated with regenerative failure in the mouse cochlea. Frontiers in Cellular Neuroscience, 9. https://doi.org/10.3389/fncel.2015.00110
- Shi, F., Kempfle, J. S., & Edge, A. S. (2012). Wnt-responsive LGR5-expressing stem cells are hair cell progenitors in the cochlea. Journal of Neuroscience, 32(28), 9639–9648. https://doi.org/10.1523/jneurosci.1064-12.2012
