Introduction to HSV-1:
Roughly 64% of people under 50 are infected with a virus called Type 1 Herpes Simplex Virus (HSV-1) [1]. One of the prominent 8 types of herpesviruses, HSV-1, is a DNA virus that currently has no complete cure. Although most people with herpes are asymptomatic or experience mild symptoms, there can be more severe side effects such as ulcers and fevers [1]. On top of this, researchers have discovered many other impacts of HSV-1 infection. Growing evidence shows that the HSV-1 pathogen is potentially associated with higher risks of Alzheimer’s Disease (AD), a dementia affecting over 55 million people [2][3].
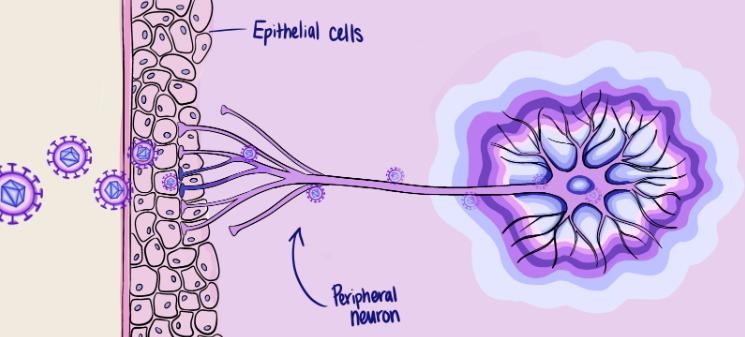
HSV-1 contains double-stranded DNA encased within a protective outer layer that both shields and assists the virus as it invades cells [4]. Proteins attached to this protective layer allow HSV-1 to enter host cells by binding these proteins to receptors on human cells, fusing its viral envelope with the cell's membrane. After HSV-1 enters the cell, the cell starts replicating its viral DNA, taking over the machinery of the cell. HSV-1 first attacks epithelial cells, such as the skin cells in and around the mouth, and then enters the peripheral neurons [4]. Studies in animals also show that, a few days after infection, HSV-1 can even reach and infect the central nervous system (CNS) [5].
Early in 1987, researchers hypothesized a method by which HSV-1 might enter CNS neurons from peripheral neurons, called retrograde transportation [6]. Generally, information is received from short branches on the neuron called dendrites, travels from the cell body through a bundle of neuron fibers called the axon and is transmitted from the axon terminal to the next neuron via a synapse, the gap between neurons. The researchers decided to investigate this by injecting HSV-1 into motor neurons, which send instructions for movement to the body's muscles. By fluorescently labeling the viral pathogens, however, they observed something surprising: HSV travels in retrograde, moving from a postsynaptic neuron's dendrites to a presynaptic neuron's axon terminals and backward to the parent cell body [6]. This finding is surprising because normally, a virus would first slip inside brain cells through the blood and then be transported in a forward path, down the neuronal axons, to affect more cells.
So why does HSV-1 exhibit a preference for infecting neurons in a retrograde manner? Retrograde transportation benefits viruses by allowing them to establish latency, enabling them to travel from a host cell to a new cell without killing the host [7]. This greatly increases the survival of HSV-1 virus inside cells, allowing it to spread across the human body and lengthening its infection period. Research suggests that this latency is mediated by the HSV-1 virus DNA [8]. In one study, researchers discovered a genetic sequence called latency-associated transcript (LAT) on HSV-1 RNA, a non-protein coding RNA that reduces HSV-1 gene transcription in the cell to build latency. By using genetic modification techniques, scientists removed the LAT sequence from HSV-1 DNA. They then combined this modified DNA with a fluorescent enzyme to easily track HSV-1 DNA expression and detect virus reactivation. The result showed that the HSV-1 DNA expression was higher in mice without LAT than in mice with LAT. Thus, researchers concluded that the LAT sequence inhibits HSV-1 reactivation and reduces HSV-1 replication [8].
Clearly, something must have occurred to activate HSV-1. Scientists hypothesize that neuron growth factors that regulate the survival and function of neurons could cause HSV-1 to reactivate [9]. Some factors such as neurturin (NTN) and glial cell line-derived neurotrophic factor (GDNF) are now being tested and for their roles in inducing HSV-1 reactivation in sensory neurons. Research shows that these factors trigger cell stress through activating an enzyme called cJun N-terminal kinase (JNK) to unwind tightly packed mRNA. This unwinding exposes mRNA’s coding area and helps translation factors sneak into the mRNA and start coding, stimulating viral gene expression [9].
Once it is reactivated, the virus will continue replicating and infecting neurons in the CNS. As it does so, it can induce brain diseases, such as HSV-1 encephalitis, a type of inflammation of the brain, and seizures. These diseases can cause neurological deficits, including cognitive, behavioral, and personality changes [9].
HSV-1 and Alzheimer's Disease
Interestingly, the effects of HSV-1 infection seem to not only be limited to these previously studied neurological deficits. A study that analyzed existing data on individuals with HSV-1 infection found that they were 2.5 times more likely to develop AD [10]. Therefore, scientists believe HSV-1 may play a role in the development or progression of AD.
AD severely affects cognitive functions, including memory, problem-solving, and abstract thinking, with substantial death of neurons as the disease progresses [11]. Recently, researchers have drawn further connections between HSV-1 and AD: specifically, that even low-level infections have the potential to create AD-like characteristics [12]. So how does low level infection of a very common virus such as HSV-1 relate to such a severe neurodegenerative disease? The answer to this question involves a protein common to both diseases, the amyloid-beta (Aβ) protein. A fundamental study done in 1992 hypothesized that abnormal processing of Aβ in neurons causes Aβ to clump together to form plaques, leading to neuron death and disrupting neuron function [13]. Since then, researchers have solidified amyloid beta as a key feature of AD, prompting ongoing studies on how various forms of the protein influence the disease, and developing treatments targeting the Aβ accumulation [14].
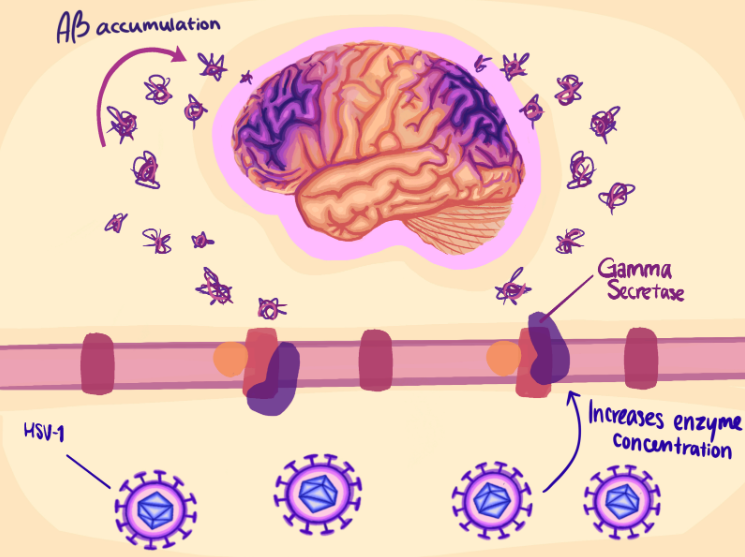
In search of treatment options for AD, researchers explored HSV-1 and its interaction with Aβ in hopes of understanding how HSV-1 and AD relate and how this could be targeted by future treatments. Current research hypothesizes that HSV-1 can increase Aβ accumulation, therefore triggering neuron death and potentially leading to the development of AD [15]. In one study, researchers used modified HSV-1 viruses with limited ability to diffuse from infected cells to surrounding tissues [15]. They injected this modified virus into certain regions of mouse brains to see how HSV-1 viruses influence specific areas of the brain. The results showed an increase in the concentration of an enzyme called gamma secretase. This enzyme cleaves proteins, including APP, the precursor to Aβ, which can create toxic byproducts that go on to form plaques when not functioning properly. In the mouse model, as more of this protein was expressed in response to HSV-1 infection, Aβ plaques were produced at a higher level in infected mice compared to the control, uninfected groups [15]. Although this experiment was performed on mice, not humans, it supports the hypothesis that HSV-1 stimulates Aβ accumulation and triggers neuron death, which can therefore contribute to AD development. In the last decades, this Aβ and HSV-1 model has been supported by a number of studies [3, 16, 17].
However, there are also many other alternative models that may explain the relationship between HSV-1 and AD. Another well-known model suggests that HSV-1 can activate the inflammatory response in neurons and potentially contribute to AD. In one study, researchers injected HSV-1 into mice and collected their brains to test how HSV-1 relates to neuronal inflammatory response [18]. They examined the expression of two mRNA sequences called SOCS2 and SOCS3, which are responsible for inhibiting the inflammatory response. Their results showed that SOCS2 and SOCS3 expression decreased with HSV-1 infection, leading to the increase in inflammatory response. They also found that the increase in neuronal inflammation led to increased neuron death. Therefore, the researchers hypothesized that HSV-1 infection activates neuron inflammatory responses that accelerate neuron death and AD development [18].
Insights from HSV-1
Although the HSV-1 virus and its effects on neurodegenerative diseases such as AD are devastating, researchers have been able to learn a lot about the mechanisms of neurodegenerative disease by studying the virus.
Scientists propose that some antiviral medicines may be helpful for treating AD. For example, the drug Valacyclovir (VCV), commonly used as an antiviral agent to treat herpes virus infections, is now being considered for treating AD. As an antiviral, it functions by targeting the DNA replication of the virus in host cells, thus preventing the virus from reproducing and spreading within neurons. A recent study investigated its efficacy in treating AD by creating a 3D bioengineered neuron model, or organoid, capable of mimicking real brain structure and hosting brain tissue cells [12]. They then infected brain cells within the model with different amounts of HSV-1 and found increased Aβ accumulation in neurons as expected. Researchers also observed that the infected cells underwent programmed neuron cell death for high level infections, while, as previously mentioned, low level infection resulted in the formation of structures similar to those in the brains of AD patients. Based on these observations, researchers took a new cell line and injected low levels of HSV-1 to the cells to emulate the neurons in AD patients infected with HSV-1. They then added VCV to this 3D tissue to test whether the drug can reduce the level of Aβ accumulation and AD-like symptoms caused by HSV-1 infection. When applied early enough, VCV reversed nearly all the changes in mediator concentrations and almost completely abolished infection. The inflammatory level was also reduced under VCV treatment [12]. The results of this study suggest that VCV has promising effects in preventing Aβ accumulation after HSV-1 infection, which implies that VCV may be a treatment option for presymptomatic AD linked to HSV-1 infection in the future [12]. However, further testing is needed to verify whether these findings are generalizable.
Other examples of medications that may treat AD-like symptoms include dexamethasone (DXMT) and acyclovir. While DXMT is an antifungal drug used clinically for allergic reactions and acyclovir is an antiviral drug, they are both used to treat herpes virus infection. A study conducted on mice co-injected with acyclovir and DXMT showed a reduction in Aβ accumulation and improvement of spatial cognition not observed when DXMT or acyclovir were given alone [19]. Therefore, these drugs could benefit some AD patients who have recently been infected by HSV-1 or are presymptomatic AD patients with recurrent latent HSV-1 infection.
Although research has shown promising results of using antibiotics to target Aβ in models of AD, its side effects remain unknown. Since the discussed research on VCV in treating AD was performed in a 3D neuron cell model, and other similar studies are done in animals, its clinical reliability is still questionable. Similarly, the effectiveness of DXMT is also controversial. The researchers’ conclusions that DXMT is successful in reducing Aβ in mice are limited by the fact that they only monitored a short-term response in the mice brains, so long-term effects of the drug are still unknown. In fact, one study claims that long-term treatment with high levels of DXMT may lead to the accumulation of toxic substances in neurons and cause cognitive impairment [20]. Therefore, using VCV and DXMT may have negative effects on the brain as well that must be further investigated before clinical use.
While much progress on understanding HSV-1 has been made in recent years, scientists are still unsure how to completely eradicate it after infection, and around 67% people remain repeatedly infected by this virus. However, HSV-1 seems to offer a valuable entry point for better understanding AD and developing preventative treatments to lower AD risk. Currently, the average life expectancy of AD is around 4 to 8 years post-diagnosis, and it is still a disease with no cure [11]. As it progresses, people with AD gradually lose the ability to respond to and engage with their environment. Simple communication may also become very hard for them, and they may act in socially inappropriate ways. In addition, many friends and even family members have a hard time taking care of them. According to reports from 2014 and 2018, 62% of female and 52% of male caregivers consider a caregiving role to be emotionally stressful [11]. AD creates heartbreaking challenges for both those with the disease and their loved ones. Hence, an effective treatment method is deeply needed. Although there is still controversy over some of the proposed treatments, such as antibiotics, that can reduce the risk of AD by preventing HSV-1 infections, these approaches still open a promising path for scientists and draw public attention to the topic of AD. Future investigation is still needed for determining the relationship between AD and HSV-1 and developing innovative treatment methods for AD.
References:
[1] World Health Organization. (n.d.). Herpes simplex virus. World Health Organization. https://www.who.int/news-room/fact-sheets/detail/herpes-simplex-virus#:~:text=Most%20people%20with%20herpes%20have,aches%20and%20swollen%20lymph%20nodes.
[2] Piacentini, R., Li Puma, D. D., Ripoli, C., Marcocci, M. E., De Chiara, G., Garaci, E., Palamara, A. T., & Grassi, C. (2015). Herpes Simplex Virus type-1 infection induces synaptic dysfunction in cultured cortical neurons via GSK-3 activation and intraneuronal amyloid-β protein accumulation. Scientific reports, 5, 15444. https://doi.org/10.1038/srep15444
[3] Adi - dementia statistics. Alzheimer’s Disease International (ADI). (n.d.). https://www.alzint.org/about/dementia-facts-figures/dementia-statistics/.
[4] Carmichael, J. C., Yokota, H., Craven, R. C., Schmitt, A., & Wills, J. W. (2018). The HSV-1 mechanisms of cell-to-cell spread and fusion are critically dependent on host PTP1B. PLoS pathogens, 14(5), e1007054. https://doi.org/10.1371/journal.ppat.1007054
[5] Doll, J. R., Thompson, R. L., & Sawtell, N. M. (2019). Infectious Herpes Simplex Virus in the Brain Stem Is Correlated with Reactivation in the Trigeminal Ganglia. Journal of virology, 93(8), e02209-18. https://doi.org/10.1128/JVI.02209-18
[6] Ugolini, G., Kuypers, H. G. J. M., & Simmons, A. (1987). Retrograde transneuronal transfer of herpes simplex virus type 1 (HSV 1) from motoneurones. Brain research, 422(2), 242-256.
[7] Miranda-Saksena, M., Denes, C. E., Diefenbach, R. J., & Cunningham, A. L. (2018). Infection and Transport of Herpes Simplex Virus Type 1 in Neurons: Role of the Cytoskeleton. Viruses, 10(2), 92. https://doi.org/10.3390/v10020092
[8] Nicoll, M. P., Hann, W., Shivkumar, M., Harman, L. E., Connor, V., Coleman, H. M., Proença, J. T., & Efstathiou, S. (2016). The HSV-1 Latency-Associated Transcript Functions to Repress Latent Phase Lytic Gene Expression and Suppress Virus Reactivation from Latently Infected Neurons. PLoS pathogens, 12(4), e1005539.
[9] Cliffe, A. R., Arbuckle, J. H., Vogel, J. L., Geden, M. J., Rothbart, S. B., Cusack, C. L., Strahl, B. D., Kristie, T. M., & Deshmukh, M. (2015). Neuronal Stress Pathway Mediating a Histone Methyl/Phospho Switch Is Required for Herpes Simplex Virus Reactivation. Cell host & microbe, 18(6), 649–658. https://doi.org/10.1016/j.chom.2015.11.007
[10] Tzeng, N. S., Chung, C. H., Lin, F. H., Chiang, C. P., Yeh, C. B., Huang, S. Y., Lu, R. B., Chang, H. A., Kao, Y. C., Yeh, H. W., Chiang, W. S., Chou, Y. C., Tsao, C. H., Wu, Y. F., & Chien, W. C. (2018). Anti-herpetic Medications and Reduced Risk of Dementia in Patients with Herpes Simplex Virus Infections-a Nationwide, Population-Based Cohort Study in Taiwan. Neurotherapeutics: the journal of the American Society for Experimental NeuroTherapeutics, 15(2), 417–429. https://doi.org/10.1007/s13311-018-0611-x
[11] 2018 Alzheimer’s disease facts and figures. (2018). Alzheimer's & dementia : the journal of the Alzheimer's Association. 14:367–429.
[12] Cairns, D. M., Rouleau, N., Parker, R. N., Walsh, K. G., Gehrke, L., & Kaplan, D. L. (2020). A 3D human brain-like tissue model of herpes-induced Alzheimer's disease. Science advances, 6(19), eaay8828. https://doi.org/10.1126/sciadv.aay8828
[13] Hardy, J. A., & Higgins, G. A. (1992). Alzheimer's disease: the amyloid cascade hypothesis. Science, 256(5054), 184-185
[14] Söderberg, L., Johannesson, M., Nygren, P., Laudon, H., Eriksson, F., Osswald, G., Möller, C., & Lannfelt, L. (2023). Lecanemab, aducanumab, and gantenerumab — binding profiles to different forms of amyloid-beta might explain efficacy and side effects in clinical trials for Alzheimer's disease. Neurotherapeutics, 20(1), 195–206. https://doi.org/10.1007/s13311-022-01308-6
[15] Zhao, M., Ma, G., Yan, X., Li, X., Wang, E., Xu, X. X., Zhao, J. B., Ma, X., & Zeng, J. (2024). Microbial infection promotes amyloid pathology in a mouse model of Alzheimer's disease via modulating γ-secretase. Molecular psychiatry, 29(5), 1491–1500. https://doi.org/10.1038/s41380-024-02428-5
[16] Qiao, H., Zhao, W., Guo, M., Zhu, L., Chen, T., Wang, J., Xu, X., Zhang, Z., Wu, Y., & Chen, P. (2022). Cerebral Organoids for Modeling of HSV-1-Induced-Amyloid β Associated Neuropathology and Phenotypic Rescue. International journal of molecular sciences, 23(11), 5981. https://doi.org/10.3390/ijms23115981
[17] Li Puma, D. D., Piacentini, R., Leone, L., Gironi, K., Marcocci, M. E., De Chiara, G., Palamara, A. T., & Grassi, C. (2019). Herpes Simplex Virus Type-1 Infection Impairs Adult Hippocampal Neurogenesis via Amyloid-β Protein Accumulation. Stem cells (Dayton, Ohio), 37(11), 1467–1480. https://doi.org/10.1002/stem.3072
[18] Toscano, E. C. B., Sousa, L. F. D. C., Lima, G. K., Mesquita, L. A., Vilela, M. C., Rodrigues, D. H., Ferreira, R. N., Soriani, F. M., Campos, M. A., Kroon, E. G., Teixeira, M. M., de Miranda, A. S., Rachid, M. A., & Teixeira, A. L. (2020). Neuroinflammation is associated with reduced SOCS2 and SOCS3 expression during intracranial HSV-1 infection. Neuroscience letters, 736, 135295. https://doi.org/10.1016/j.neulet.2020.135295
[19] Hui, Z., Zhijun, Y., Yushan, Y., Liping, C., Yiying, Z., Difan, Z., Chunglit, C. T., & Wei, C. (2020). The combination of acyclovir and dexamethasone protects against Alzheimer's disease-related cognitive impairments in mice. Psychopharmacology, 237(6), 1851–1860. https://doi.org/10.1007/s00213-020-05503-1
[20] Anderson, G., & Ojala, J. (2010). Alzheimer's and seizures: interleukin-18, indoleamine 2,3-dioxygenase and quinolinic Acid. International journal of tryptophan research: IJTR, 3, 169–173. https://doi.org/10.4137/IJTR.S4603