Introduction
Imagine a silent thief that slowly and methodically robs you of one of your most precious senses: sight. This is glaucoma, a neurodegenerative disease that affects 80 million people worldwide and can cause irreversible blindness before its victims even realize their vision is deteriorating. Glaucoma often leads to peripheral vision loss, and patients commonly report needing more light and experiencing blurred vision [1]. Without obvious symptoms in its early stages, glaucoma often remains undetected until significant vision loss has occurred.
Glaucoma can manifest in different forms, classified as primary or secondary glaucoma. Primary glaucoma occurs on its own, while secondary glaucoma is induced by other medical conditions and is significantly rarer. There are four main types of primary glaucoma: open-angle glaucoma, normal-tension glaucoma, angle-closure glaucoma, and congenital glaucoma [2]. Among these, primary open-angle glaucoma (POAG) is the most common, affecting around 90% of glaucoma patients and occurring when the optic nerve is damaged due to increased eye pressure. In normal-tension glaucoma, patients develop optic nerve damage without increased eye pressure. Angle-closure glaucoma occurs when fluid drainage is blocked by the outer edge of the iris, causing a sudden increase in eye pressure and potentially leading to blindness within days if untreated. Finally, congenital glaucoma occurs in infants whose eyes cannot drain fluid properly [2].
Across the varied forms of this disease, glaucoma’s underlying mechanisms show striking similarities to those found in other neurodegenerative disorders, particularly Alzheimer’s disease (AD). Research shows that glaucoma shares key pathological features and has genetic overlap with AD, positioning it as a valuable lens through which we can study brain health. Though no cure exists, recent studies are exploring treatments beyond lowering eye pressure, aiming to protect the optic nerve and potentially target shared pathways with neurodegenerative diseases.
What is glaucoma?
Glaucoma is a chronic eye condition that damages the optic nerve, the part of the eye responsible for transmitting visual information to the brain [3]. More specifically, glaucoma causes neurodegeneration of the optic nerve and leads to the loss of retinal ganglion cells (RGCs) in the retina [3]. The retina is the innermost layer of the eye and, with the help of the brain, works to transform light energy from photons into three-dimensional images, essentially creating what we perceive as “sight.” RGCs are the retina’s main output neurons, meaning that signals travel through the RGCs before they reach the brain, where this visual information is then received and processed [4]. RGC axons bundle together to form the optic nerve, which exits the eye by passing through the optic nerve head (ONH) [3]. As glaucoma progresses, the neural rim of tissue on the ONH formed by the exiting RGC axons gradually thins, leading to a characteristic “cupped” appearance [3].
While the exact cause of glaucoma is unknown, several risk factors contribute to its progression. These include increased age, elevated intraocular pressure (IOP), near-sightedness, and a positive family history of glaucoma. Normal eye pressure ranges between 10-21 mmHg, with levels exceeding 21 mmHg typically associated with glaucoma [5]. Currently, high IOP, which occurs when fluid is unable to drain normally out of the front of the eye, is the only known modifiable risk factor for open-angle glaucoma [6]. Numerous studies and clinical trials have demonstrated that reducing IOP significantly impacts glaucoma progression and prevention. However, despite the success of treatments targeting IOP, glaucoma’s progression often continues, suggesting that IOP is just one piece of a much larger puzzle [7].
Generally, the progression of the disease is slow and relatively painless, and most patients with glaucoma do not notice vision problems until significant vision loss has already occurred. One-third of patients are diagnosed at advanced stages, with significant visual field loss that impairs them from daily tasks and activities such as driving [6][8]. This development pattern of glaucoma parallels other neurodegenerative diseases like AD, where damage often accumulates long before symptoms emerge.
Common pathways with other neurodegenerative disorders
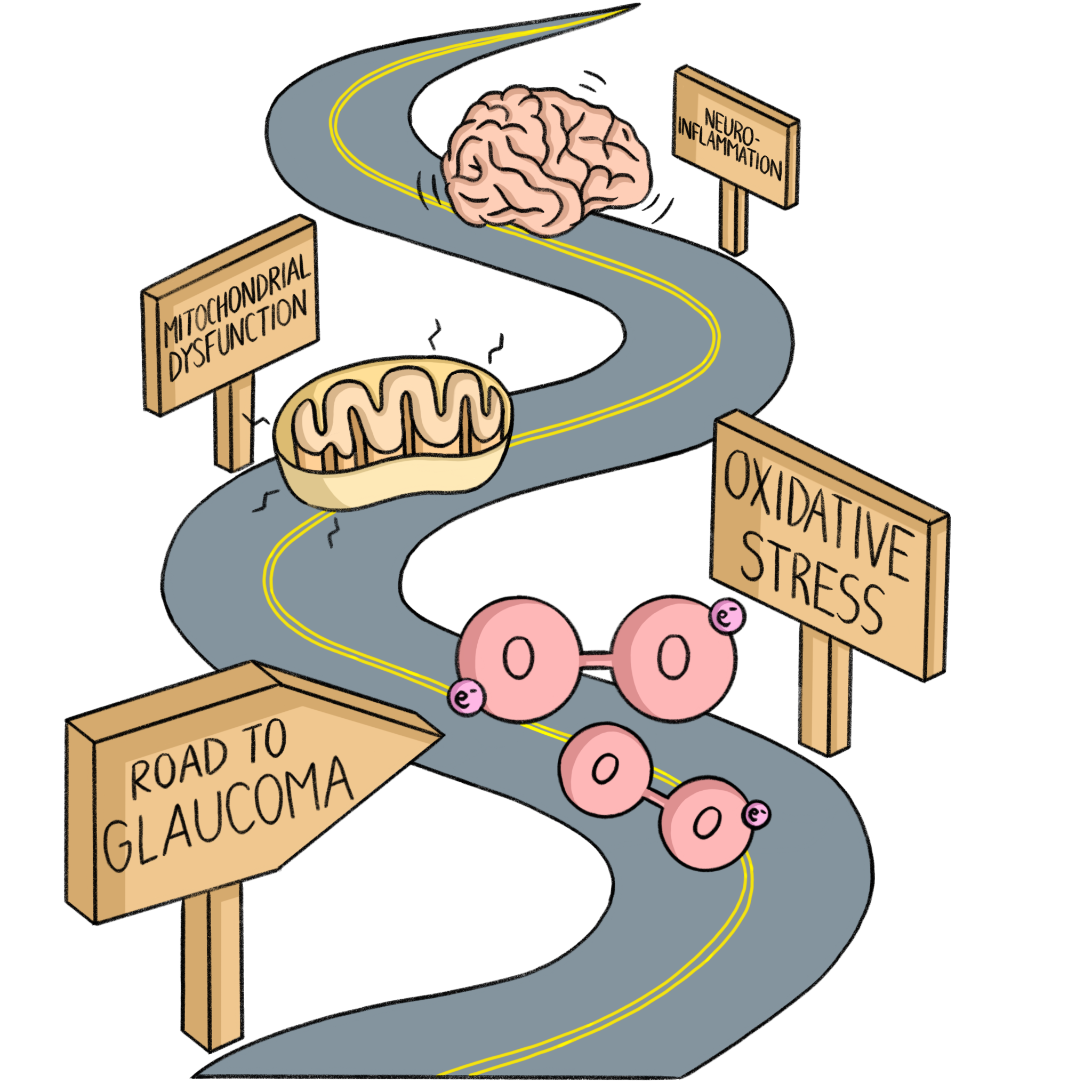
Although glaucoma is primarily a retinal disease, it shares many underlying mechanisms with other neurodegenerative disorders. This article primarily focuses on discussing commonalities between glaucoma and AD, since AD is the most common neurodegenerative disorder. These mechanisms – such as oxidative stress, mitochondrial dysfunction, and neuroinflammation – are central to the progression of both conditions. Understanding these shared processes not only provides insight into their overlapping biology but also opens the door to potential cross-disease strategies for intervention.
Oxidative Stress
Oxidative stress, a major driver of neurodegeneration, is pivotal in the progression of glaucoma. Oxygen, due to its atomic structure and two unpaired electrons, is prone to becoming a free radical called a reactive oxygen species (ROS), a highly unstable and reactive molecule. When an imbalance between the production of ROS and the body’s ability to neutralize them occurs, it leads to oxidative stress, causing cellular damage, mitochondrial dysfunction, and eventual cell death as the oxidative burden overwhelms antioxidant defenses [9].
In glaucoma, oxidative stress plays a direct role in the degeneration of RGCs. ROS-induced damage is believed to contribute to both elevated IOP and RGC death, exacerbating vision loss [10]. This damaging effect of oxidative stress is evident in both POAG and normal-tension glaucoma, where RGC death can occur even without significant increase in IOP. A study found that open-angle glaucoma patients had significantly lower systemic antioxidant capacity compared to healthy controls. Specifically, lower levels of biological antioxidant potential, a marker of antioxidant defense, were associated with more severe visual field damage in these patients [10].
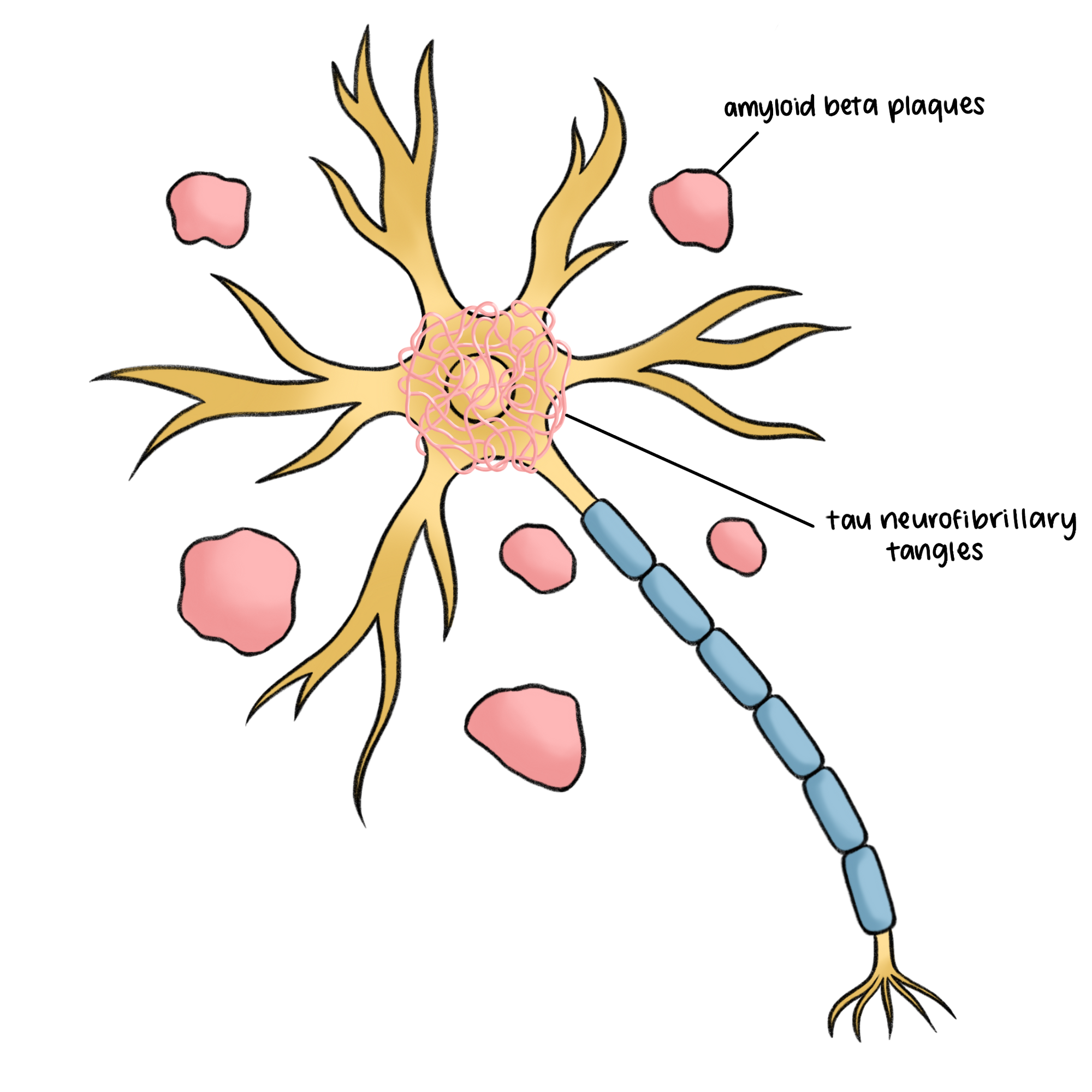
Similarly, in AD, oxidative stress is a contributing factor to the buildup of tau neurofibrillary tangles and amyloid beta (Aβ) plaques – two key pathological markers that cause neuronal death in AD [11]. Tau proteins, normally essential for stabilizing neuron structure, become chemically altered through hyperphosphorylation, a process where excess phosphate groups attach to proteins, impairing their normal function. This modification causes tau proteins to twist into neurofibrillary tangles, which block nutrient transport within the cell and lead to cell death. Aβ plaques form when protein fragments cluster between neurons, interrupting cell communication and sparking an inflammatory response that damages surrounding cells. Aβ plaques disrupt calcium (Ca2+) storage in the endoplasmic reticulum, an organelle responsible for protein and lipid synthesis and calcium storage, causing cytosolic Ca2+ overload and oxidative stress. Excessive ROS contributes to the accumulation of Aβ plaques and triggers cellular pathways leading to tau hyperphosphorylation and cell death. This cycle of oxidative damage exacerbates mitochondrial dysfunction, leading to further ROS production, reduced adenosine triphosphate (ATP) levels, and Ca2+ imbalance, which are implicated in AD progression [11]. Elevated ROS levels drive neurodegeneration in both AD and glaucoma [9].
Mitochondrial Dysfunction
Mitochondrial dysfunction and oxidative stress create a damaging cycle in which impaired mitochondria produce excessive ROS, leading to oxidative stress that further damages mitochondria, ultimately amplifying cellular degeneration in both glaucoma and AD [12]. Neurons, especially those in the eye, rely heavily on energy produced by mitochondria to function properly. In glaucoma, when eye pressure rises, mitochondria produce less energy (ATP), which affects how well nerve signals are transmitted. This decrease in ATP happens before noticeable damage to the nerve structure occurs. Along with low energy levels, mitochondria also produce ROS, which cause further damage and stress to the cells. The damage from these ROS can lead to cell death through a process called apoptosis. While blocking cell death might slow the damage, it does not stop the overall loss of vision in glaucoma. In AD, mitochondrial dysfunction plays a critical role in the formation of toxic plaques and tangles, further contributing to neuronal loss. The failure of mitochondria to maintain energy production in the face of oxidative stress exacerbates the neurodegeneration observed in both AD and glaucoma [12].
Neuroinflammation
Neuroinflammation, the process of immune system activation in the brain in response to injury, is also a shared pathway between glaucoma and AD. Growing evidence suggests that neuroinflammation plays a significant role in both the early stages and progression of the disease. Initially, neuroinflammatory responses may have protective effects aimed at restoring homeostasis between RGCs and their environment. However, chronic inflammation involving overproduction of chemokines, cytokines, and activation of innate immunity can lead to RGC degeneration [13]. Recent evidence indicates that neuroinflammation is also central to AD progression. Microglial activation, a process in which microglia, immune cells in the brain, change in response to brain injuries, is a key component of neuroinflammation. This activation interacts with Aβ and tau pathologies, influencing AD’s progression and offering a potential target for new therapeutic strategies [14].
While glaucoma primarily affects the eyes, its mechanisms of oxidative stress, mitochondrial dysfunction, and neuroinflammation reflect broader patterns of neurodegeneration seen in AD. These common pathways not only illustrate the pathology but also point to potential therapeutic targets that could benefit a range of neurodegenerative conditions. Understanding the links between glaucoma and these disorders could help develop strategies for early detection and intervention, potentially slowing progression in both the eye and the brain [15].
Genetic Overlap
The relationship between glaucoma and neurodegenerative diseases extends beyond shared pathways– there are also common genetic components. Recent studies have begun to untangle the genetic groundwork that links glaucoma with conditions like AD. By examining genetic data from extensive research cohorts, researchers are uncovering common genetic variants that may influence the susceptibility to both glaucoma and other neurodegenerative diseases. This genetic overlap not only highlights the interconnectedness of these conditions but also offers insights into the shared biological pathways that govern the health of both the eye and the brain. Understanding these genetic relationships could pave the way for new diagnostic and therapeutic approaches that address the complexities of these disorders.
One study investigated the genetic connections between POAG and the major neurodegenerative diseases, including AD [16]. Genome-wide association data from studies of magnetic resonance imaging studies on brain structures were utilized to see if there were shared genetic factors between POAG and AD, and if one condition might influence the development of another. The researchers found that certain genetic traits, like those in the MAPT gene region on chromosome 17, are involved in both AD and glaucoma. The MAPT gene is known for its role in producing tau protein, which can form harmful clumps in the brains of AD patients. This gene may also influence the optic nerve and the retina, which are affected in glaucoma. Additionally, researchers noticed a relationship between IOP and brain structures. The frontal pole, the part of the frontal lobe involved in decision making, and grey matter volume, general brain tissue density, are genetically correlated with IOP, meaning certain genes may impact both eye pressure and brain structure. Furthermore, retinal markers for glaucoma, such as the macular retinal nerve fiber layer and the ganglion cell-inner plexiform layer, were associated with structural changes in areas like the parahippocampal region, which may signal shared neurodegenerative pathways. The findings suggest that vascular or inflammatory processes could underlie both diseases, opening avenues for further research on their intertwined nature [16].
In another study, researchers examined the association between open-angle glaucoma (OAG) and the subsequent risk of developing neurodegenerative diseases, specifically AD [17]. The study tracked the health outcomes of individuals diagnosed with OAG over a 10-year follow-up period. The results indicated a significant increase in the risk of developing AD among OAG patients, particularly in those older than 65 years [17]. Another comprehensive systematic review and meta-analysis of eight observational studies also investigated the relationship between glaucoma and the risk of developing AD. The findings revealed that individuals with glaucoma had a 1.52-fold increased risk of developing AD compared to those without the condition [18]. These findings underscore the potential link between OAG and an elevated risk of AD, suggesting that early detection and management of glaucoma may play a role in mitigating the progression of neurodegenerative diseases.
Implications for treatment and research
Recent research has begun to explore the implication of the intricate connections between AD and glaucoma, shedding light on potential new treatment strategies. By targeting specific pathways involved in neuroinflammation and cell health, researchers hope to develop therapies that could improve outcomes for individuals facing both cognitive impairment and visual deterioration, emphasizing the need for an integrated approach to these interconnected disorders.
A recent study explored the role of the APOE4 allele, which is associated with a higher risk of AD but a lower risk of glaucoma [19]. Researchers found that in glaucoma, microglia, which are immune cells in the brain and a key component of AD, shift to a neurodegenerative state, leading to increased levels of Apoe and Galectin-3, two proteins which are toxic at high levels. However, mice with the disease-associated human APOE4 allele or lacking the Apoe gene entirely did not show the harmful effects of this transition, as their microglia did not activate these toxic proteins despite elevated eye pressure. Interestingly, when researchers targeted Galectin-3, either through genetic manipulation or with drugs, they found protection from RGC loss, a key factor in glaucoma-induced vision loss. This suggests that the APOE4 allele might protect against glaucoma damage by preventing microglial activation and the production of toxic proteins. Additionally, human glaucoma samples with the APOE4 allele exhibited lower Galectin-3 levels compared to those with the more common APOE3 allele. These findings highlight the potential of targeting the Apoe-Galectin-3 signaling pathway as a novel treatment strategy for glaucoma. This could provide a new therapeutic approach to preserving vision and offer deeper insights into the genetic mechanisms underlying glaucoma [19].

Another study investigated the use of two FDA-approved carbonic anhydrase inhibitors (CAIs) methazolamide and acetazolamide, to see if they could help prevent cognitive decline in AD and cerebral amyloid angiopathy (CAA), which occurs when Aβ plaques build up in arteries in the brain [20]. These drugs, which are typically used to treat glaucoma and high altitude sickness, helped improve brain function in mice with genetically-induced AD by reducing the accumulation of Aβ plaques. The CAIs also improved brain blood vessel health, decreased damaging cellular processes, and promoted the clearance of Aβ, which reduced inflammation and boosted cell function. This study showed that long term treatment with these drugs may decrease Aβ deposits across different brain regions, including the hippocampus, cortex, and hypothalamus. The CAIs also reduced other harmful effects on brain cells, such as excessive caspase activation, a marker for cell death, and gliosis, cellular inflammation. Importantly, the drugs did not cause toxicity in the mice. In behavioral tests, the treated mice performed similarly to healthy control mice in tasks related to motor coordination and memory, suggesting that these drugs could even reverse some of the cognitive impairments caused by AD. The researchers also showed that the drugs improved the function of microglia and astrocytes, which are supportive brain cells that are crucial for clearing away Aβ. When these cells are stressed or damaged in AD, their ability to remove Aβ is impaired, but CAIs help restore their normal function, improving overall brain health. The CAIs also prevented mitochondrial dysfunction, which is often triggered by the accumulation of Aβ, thus protecting brain cells from death. In conclusion, the potential of CAIs to treat AD and CAA highlights the link between glaucoma medications and neurodegenerative disease therapies, demonstrating how treatments for one condition could offer new solutions for others [20].
Conclusion
As we unravel the mysteries of glaucoma, we begin to see it not just as a thief lurking in the shadows, but as a complex player in the broader narrative of neurodegeneration. This condition stealthily robs individuals of their vision, leaving its victims unaware of the damage until significant vision loss occurs. Yet, as we dig deeper, we uncover that this thief’s reach extends beyond sight. Glaucoma's intricate relationship with other neurodegenerative diseases like AD reveals that it may be part of a larger, interconnected web of neurological decline. These diseases share common traits, whether it is the breakdown of neuronal pathways or the disruption of cellular function, suggesting that the thief’s motives may not be isolated to vision alone, but rather a broader assault on our nervous system. By exploring these relationships, researchers can shine a light on shared mechanisms, paving the way for innovative treatments that may disarm this thief from multiple angles. Understanding glaucoma as part of this larger story encourages a more comprehensive approach to tackling both vision loss and neurodegenerative challenges. In the quest to outsmart the thief, the insights gained can lead us to a brighter, clearer future for those affected.
References
- Hu, C. X., Zangalli, C., Hsieh, M., Gupta, L., Williams, A. L., Richman, J., & Spaeth, G. L. (2014). What do patients with glaucoma see? Visual symptoms reported by patients with glaucoma. The American journal of the medical sciences, 348(5), 403–409. https://doi.org/10.1097/MAJ.0000000000000319
- Dietze, J., Blair, K., Zeppieri, M., & Havens, S. J. (2024). Glaucoma. In StatPearls. StatPearls Publishing.
- Nickells, R. W., Howell, G. R., Soto, I., & John, S. W. (2012). Under pressure: cellular and molecular responses during glaucoma, a common neurodegeneration with axonopathy. Annual review of neuroscience, 35, 153–179. https://doi.org/10.1146/annurev.neuro.051508.135728
- Mahabadi, N., & Al Khalili, Y. (2023). Neuroanatomy, Retina. In StatPearls. StatPearls Publishing.
- Wang, Y. X., Xu, L., Wei, W. B., & Jonas, J. B. (2018). Intraocular pressure and its normal range adjusted for ocular and systemic parameters. The Beijing Eye Study 2011. PloS one, 13(5), e0196926. https://doi.org/10.1371/journal.pone.0196926
- Schuster, A. K., Erb, C., Hoffmann, E. M., Dietlein, T., & Pfeiffer, N. (2020). The Diagnosis and Treatment of Glaucoma. Deutsches Arzteblatt international, 117(13), 225–234. https://doi.org/10.3238/arztebl.2020.0225
- Jayaram H. (2020). Intraocular pressure reduction in glaucoma: Does every mmHg count?. Taiwan journal of ophthalmology, 10(4), 255–258. https://doi.org/10.4103/tjo.tjo_63_20
- Gramer, G., & Gramer, E. (2018). Stage of visual field loss and age at diagnosis in 1988 patients with different glaucomas: implications for glaucoma screening and driving ability. International ophthalmology, 38(2), 429–441. https://doi.org/10.1007/s10792-017-0477-7
- Teleanu, D. M., Niculescu, A. G., Lungu, I. I., Radu, C. I., Vladâcenco, O., Roza, E., Costăchescu, B., Grumezescu, A. M., & Teleanu, R. I. (2022). An Overview of Oxidative Stress, Neuroinflammation, and Neurodegenerative Diseases. International journal of molecular sciences, 23(11), 5938. https://doi.org/10.3390/ijms23115938
- Tanito, M., Kaidzu, S., Takai, Y., & Ohira, A. (2016). Association between systemic oxidative stress and visual field damage in open-angle glaucoma. Scientific reports, 6, 25792. https://doi.org/10.1038/srep25792
- Liu, Z., Zhou, T., Ziegler, A. C., Dimitrion, P., & Zuo, L. (2017). Oxidative Stress in Neurodegenerative Diseases: From Molecular Mechanisms to Clinical Applications. Oxidative medicine and cellular longevity, 2017, 2525967. https://doi.org/10.1155/2017/2525967
- Jassim, A. H., Inman, D. M., & Mitchell, C. H. (2021). Crosstalk Between Dysfunctional Mitochondria and Inflammation in Glaucomatous Neurodegeneration. Frontiers in pharmacology, 12, 699623. https://doi.org/10.3389/fphar.2021.699623
- Williams, P. A., Marsh-Armstrong, N., Howell, G. R., & Lasker/IRRF Initiative on Astrocytes and Glaucomatous Neurodegeneration Participants (2017). Neuroinflammation in glaucoma: A new opportunity. Experimental eye research, 157, 20–27. https://doi.org/10.1016/j.exer.2017.02.014
- Leng, F., & Edison, P. (2021). Neuroinflammation and microglial activation in Alzheimer disease: where do we go from here? Nature reviews. Neurology, 17(3), 157–172. https://doi.org/10.1038/s41582-020-00435-y
- Sen, S., Saxena, R., Tripathi, M., Vibha, D., & Dhiman, R. (2020). Neurodegeneration in Alzheimer's disease and glaucoma: overlaps and missing links. Eye (London, England), 34(9), 1546–1553. https://doi.org/10.1038/s41433-020-0836-x
- Diaz-Torres, S., He, W., Thorp, J., Seddighi, S., Mullany, S., IGGC International Glaucoma Genetics Consortium, Hammond, C. J., Hysi, P. G., Pasquale, L. R., Khawaja, A. P., Hewitt, A. W., Craig, J. E., Mackey, D. A., Wiggs, J. L., van Duijn, C., Lupton, M. K., Ong, J. S., MacGregor, S., & Gharahkhani, P. (2023). Disentangling the genetic overlap and causal relationships between primary open-angle glaucoma, brain morphology and four major neurodegenerative disorders. EBioMedicine, 92, 104615. https://doi.org/10.1016/j.ebiom.2023.104615
- Moon, J. Y., Kim, H. J., Park, Y. H., Park, T. K., Park, E. C., Kim, C. Y., & Lee, S. H. (2018). Association between Open-Angle Glaucoma and the Risks of Alzheimer's and Parkinson's Diseases in South Korea: A 10-year Nationwide Cohort Study. Scientific reports, 8(1), 11161. https://doi.org/10.1038/s41598-018-29557-6
- Xu, X. H., Zou, J. Y., Geng, W., & Wang, A. Y. (2019). Association between glaucoma and the risk of Alzheimer's disease: A systematic review of observational studies. Acta ophthalmologica, 97(7), 665–671. https://doi.org/10.1111/aos.14114
- Margeta, M. A., Yin, Z., Madore, C., Pitts, K. M., Letcher, S. M., Tang, J., Jiang, S., Gauthier, C. D., Silveira, S. R., Schroeder, C. M., Lad, E. M., Proia, A. D., Tanzi, R. E., Holtzman, D. M., Krasemann, S., Chen, D. F., & Butovsky, O. (2022). Apolipoprotein E4 impairs the response of neurodegenerative retinal microglia and prevents neuronal loss in glaucoma. Immunity, 55(9), 1627–1644.e7. https://doi.org/10.1016/j.immuni.2022.07.014
- Canepa, E., Parodi-Rullan, R., Vazquez-Torres, R., Gamallo-Lana, B., Guzman-Hernandez, R., Lemon, N. L., Angiulli, F., Debure, L., Ilies, M. A., Østergaard, L., Wisniewski, T., Gutiérrez-Jiménez, E., Mar, A. C., & Fossati, S. (2023). FDA-approved carbonic anhydrase inhibitors reduce amyloid β pathology and improve cognition, by ameliorating cerebrovascular health and glial fitness. Alzheimer's & dementia: the journal of the Alzheimer's Association, 19(11), 5048–5073. https://doi.org/10.1002/alz.13063