Introduction
Imagine you’re at a college party: The music’s loud, people are dancing, and you’re surrounded by bottles of whiskey, beer, vodka, and rum. You take a shot, and someone hands you another drink. As you down your second shot, the alcohol moves into the stomach and enters your bloodstream. Vigorously pumped through the blood, it travels throughout your body, entering your liver, your pancreas, and your brain [1]. The brain is highly protected from foreign agents by a layer called the blood-brain barrier, but alcohol can diffuse through it because it is fat-soluble [2].
Once in the brain, alcohol can have many effects. In this article, we’ll explore how our neuroimmune systems can impact the amount of alcohol someone drinks, how acute drinking and long-term alcohol consumption can affect the brain, and why drinking is particularly dangerous for adolescents.
Neuroimmune System
The nervous system is particularly delicate compared to other systems of the body and requires significant protection [2]. The meninges, layers of protective tissue located underneath the skull, prevent unwanted or excessive fluids and molecules from entering the brain. The immune system’s main purpose is the destruction of bacterial and viral infections. Through this process, the immune system also sometimes destroys the body’s own cells in order to completely eradicate infections. To prevent this from occurring in the brain, a part of the body that has difficulty repairing itself, the brain barricades itself from the immune system and blocks many foreign molecules or substances from entering the brain [2]. Generally, immune cells can only enter the brain when there is neuroinflammation, characterized by the activation of the immunological properties of glial cells, support cells of neurons [3]. The neuroimmune response is the trafficking of immune cells into the brain, the assembly of cytokines by the brain, and the participation of immune cells in the removal of synapses and plasticity of the brain [2][4].
The immune system includes both the innate immune response and the adaptive immune response [5]. The innate immune response is immediate and non-selectively targets pathogens, whereas the adaptive immune response targets specific pathogens that the body has already been exposed to [6]. Some innate immune cells check for infections and then activate the immune response, and others capture pathogens and help create antibodies [5][7]. The immune response releases cytokines, leading to the activation of more immune cells. This activates the adaptive immune response, which involves T and B cells. T helper cells activate monocytes, cytotoxic T cells, and B cells. Cytotoxic T cells destroy cells infected with the pathogen and tumor cells. B cells become plasma cells that produce antibodies that eliminate pathogens [5].
Mechanism of Alcohol Entering the Brain
When foreign substances enter the body, they attach to small proteins on immune cells called toll-like receptors (TLRs), which activate the immune response through the production of cytokines [8]. TLRs are also expressed in neurons and glia, and their activation induces neuroinflammation [8]. Different TLRs respond to different bacterial and viral components to effectively target specific pathogens [9]. For example, TLR4 is typically activated by lipopolysaccharides, endotoxins released when a bacteria cell dies [9].
Ethanol, the active component of alcohol beverages, affects the functioning of some types of TLRs, primarily through two specific pathways: one designed for detecting bacterial infections (MyD88 pathway), and one that responds to viruses (TRIF pathway) [10]. The MyD88 pathway releases interleukin 1 beta (IL-B), interleukin 6 (IL-6), and tumor necrosis factor alpha (TNFα) [11]. The interleukins trigger the differentiation, activation, and reproduction of immune cells, and TNFα regulates cell survival and proliferation [12]. These cytokines have an amplifying effect, causing the release of more cytokines, thereby activating more immune cells and furthering the response [13]. On the other hand, the TRIF pathway releases interferons, which prevent cells infected with viruses from replicating [14].
When a foreign particle, such as alcohol or a pathogen, is in the vicinity of a TLR receptor, a specific co-receptor called cluster of differentiation 14 (CD14) binds to the foreign particle [15]. The CD14 and foreign particles then bind to the TLR, initiating the MyD88 and/or TRIF pathway, which both result in the production of cytokines [16]. Most TLRs only express the adaptor protein MyD88, but TLR3 only utilizes a TRIF pathway and TLR4 utilizes both. As such, altering the expression of these different TLRs can give insight into the mechanisms of how the neuroimmune system may affect alcohol consumption [10][16].
Researchers at the University of Texas knocked out (KO) CD14, MyD88, and some of the TLR genes in mice to test their effects on alcohol consumption. [10]. Genes that are knocked out do not express a protein. By the KO of these genes, researchers could identify how specific proteins function and affect alcohol consumption. The researchers discovered the knockout of CD14 resulted in a decrease in alcohol consumption. The KO of MyD88 led to increased consumption, and the KO of TLR4 showed no effects on consumption. These results indicate that the TRIF pathway may be involved in binge-drinking behavior because it is one of the only pathways that would be affected by the loss of CD14 besides those of MyD88, whose KO increased rather than decreased alcohol consumption. Since the MyD88 pathway is activated by all TLRs pathways except the TLR3, the increased alcohol consumption when such pathways were knocked out suggests MyD88 is a negative regulator of the TRIF pathway, with its activation decreasing binge drinking. Interestingly, results differed depending on the sex of the mouse, warranting a need for further investigation into how sex may influence alcohol consumption [10].
Other studies are consistent with this finding, showing TLR2 and TLR3 have an effect on binge drinking. The activation of TLR3, with only the TRIF pathway, has been shown to increase alcohol binge drinking [17]. Some findings, however, are not fully consistent with the previous studies. For example, deletion of TLR2, which only has the MyD88 pathway, resulted in decreased consumption of alcohol [17]. While this supports the hypothesis that TLR4 does not have an effect on binge drinking, it contradicts the previous experiment at the University of Texas, which showed that blocking of the MyD88 through the removal of proteins increased alcohol consumption [10]. This demonstrates that the pathways alcohol affects are more nuanced than previously thought, with different pathways affecting different alcohol responses [18].
Acute Effects of Alcohol on the Neuroimmune System
The previous findings demonstrate that the neuroimmune system affects alcohol drinking. Additionally, alcohol has also been shown to impact cytokine levels in the brain in several ways, causing inflammation. With increased drinking, the permeability of the intestines increases and allows for greater flow of bacteria and the endotoxins they release into the bloodstream [19]. These endotoxins then induce an inflammatory response in the liver, resulting in the production of inflammatory cytokines such as TNF-a, IL-1b, and IL-6 [20]. The vagus nerve detects these inflammatory responses through cytokine receptors and activates the production of cytokines in the brain, inducing a sickness response. The inflammatory cytokines produced in the liver can also travel through the bloodstream and enter the brain through weak spots in the blood-brain barrier [20]. Ethanol also directly enters the brain, activating neurons and the TLRs of microglia. This activation ultimately leads to an even greater release of cytokines, which have receptors on neurons and glia [18][21].
According to the National Institute on Alcohol Abuse and Alcoholism, in humans, a binge dose is “a pattern of drinking alcohol that brings the blood alcohol concentration (BAC) to 0.08 percent– or 0.08 grams of alcohol per deciliter– or higher. For a typical adult, this pattern corresponds to consuming 5 or more drinks (male), or 4 or more drinks (female), in about 2 hours”[1]. Interestingly, one study found that acute binge-like doses of ethanol in the brains of adult rats led to a particular pattern of cytokine release, notably, an increase in IL-6 and a decrease in TNFα and IL-1β [22]. These changes, observed a few hours after ethanol injection, have been termed “rapid alterations in neuroimmune gene expression” (RANGE). TNFa and IL-1b are inflammatory cytokines and their reduction from baseline levels causes a dampened immune response [5]. This suggests that at about 2-5 hours after a binge alcohol dose, alcohol produces a inhibitory effect on the innate immune system [22].
However, this pattern of cytokine release changes the longer ethanol is within the body. One study using mice, for example, measured the concentrations of key cytokines 24 hours after ethanol injection [23]. After 24 hours, TNFα mRNA increased in the hippocampus, a region of the brain involved in learning and memory, about 4 times compared to controls. Another study extended this timeline to 48 hours after an acute injection of ethanol, observing the elevation of TNFα, IL-1β, and IL-6 in the hypothalamus, a region in the brain that maintains homeostasis [24][25]. This increase in cytokines is observed even after all the ethanol has exited the bloodstream [25]. IL-6 exhibits both inflammatory and anti-inflammatory properties. The anti-inflammatory responses may activate when ethanol is in the brain and account for the initial reduction of TNFα and IL-1β [26]. After this initial response, IL-6 may interact with other feedback loops, eventually leading to increased activation of TNFα and IL-1β [26].
Adolescents, however, have been shown to have a more muted neuroimmune response compared to adults [18]. TLR4 senses gram-negative bacterial lipopolysaccharide (LPS), a bacterial toxin that activates cytokines [9][27]. One study compared cytokine levels in both adolescent and adult rats injected with either bacterial LPS or ethanol [26]. LPS is an immune agent and serves as a positive control. Notably, both LPS and ethanol injection resulted in enhanced activation of cytokines in both adolescent and adult rats, with LPS having a stronger effect than ethanol. A combination of ethanol and LPS injected resulted in a much larger increase in cytokines than LPS alone. However, this response was less extreme in adolescent rats, supporting the idea that adolescents may have a dampened neuroimmune response to alcohol consumption [26].
On the macroscopic level, the influx of IL-1β, IL-6, and TNFα induce “sickness behaviors,” which include fever, reduced water, decreased appetite, inability to concentrate, reduced activity, and sleepiness [28]. Alcohol withdrawal or hangovers have similar symptoms, and have been associated with changes in cytokine levels in several studies in humans as well as animals [29]. Studies do vary on when the cytokine levels were checked after alcohol consumption and what particular cytokine levels are increased. For instance, in one study in adult male humans, hangover states have been correlated with increases in IL-6, IL-10, IL-12, and IFNy 13 hours after alcohol consumption [30][31].
There is a positive association between cytokine levels and memory loss, which typically occurs during alcohol hangovers [32]. When an endotoxin was introduced into the bloodstream to induce IL-1b, IL-6, and TNFa, memory was affected during a word learning and story recall test. This further demonstrates the influx of cytokines due to alcohol consumption plays a role in the symptoms of a hangover [32].
There are other factors beside cytokines that cause symptoms of a hangover after alcohol consumption [33]. Dehydration occurs because alcohol causes the body to increase urinary output and low blood sugar occurs because alcohol disrupts the processes of the liver. Additionally, alcohol causes poor quality of sleep and the general disruption of circadian rhythms [33].
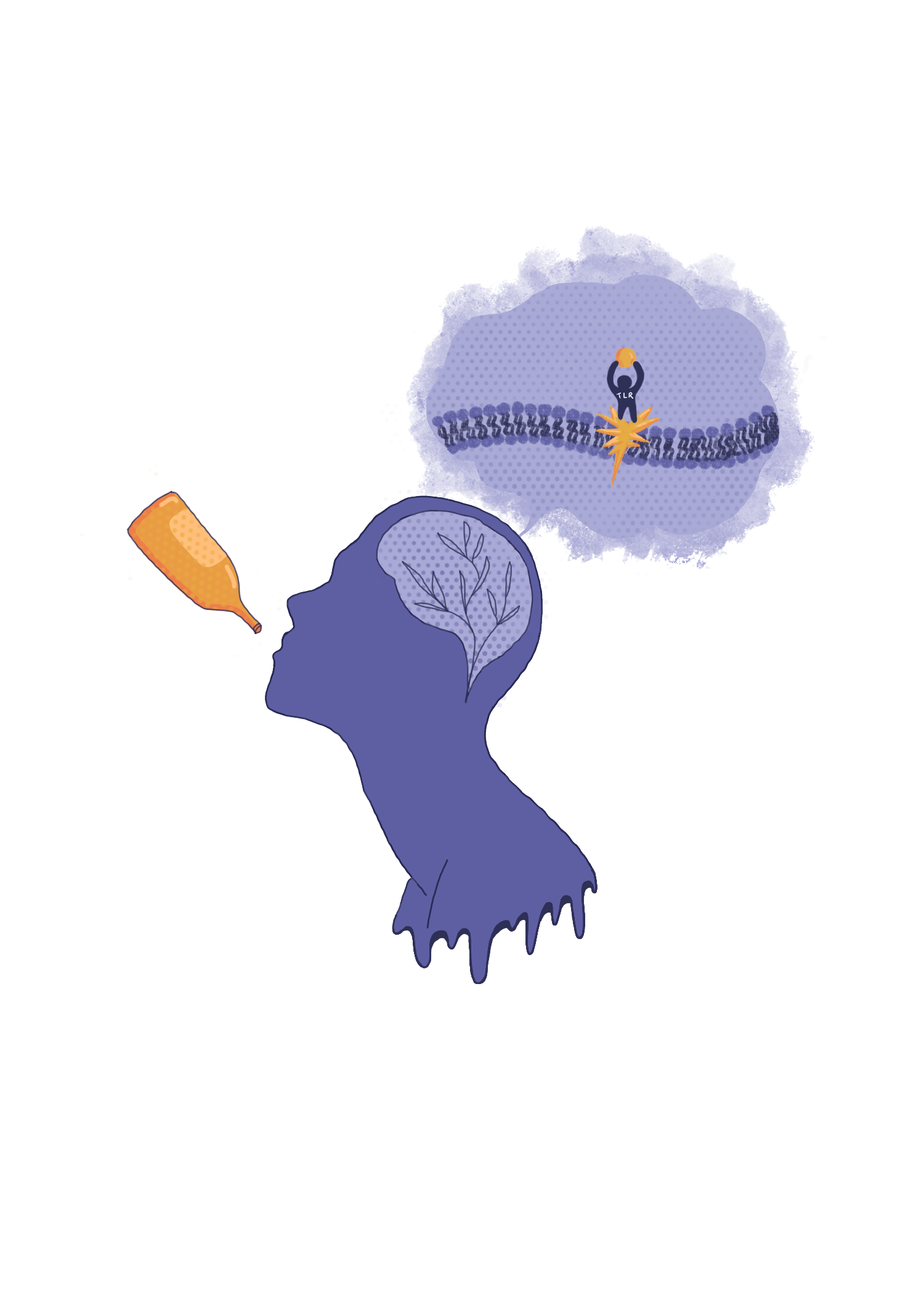
Chronic Effects of Ethanol Consumption
While we have shown the acute effects of ethanol, alcohol has much more severe effects when it is consumed more consistently. Chronic alcohol use in adults has been reported to cause behavioral and cognitive deficits, impair immune system responses, and induce neuroinflammatory brain damage [18]. In adolescents, alcohol use has similar, but more intense effects [18]. In addition, adolescents are more likely to become addicted to alcohol [34].
Research into the effects of chronic alcohol consumption on the neuroimmune system provides more insight into the long-term effects of consistent drinking [3]. In one study, female mice had their water replaced with 10% ethanol with a solid diet for 5 months, finding TLR4 to be necessary for the elevated levels of various cytokines. Similar to the increase after acute consumption, after 5 months of this treatment the mice had significantly increased levels of MyD88 and CD14, as well as the cytokines TNFa, IL-1B, and IL-6 mRNA. The mRNA of TLR4 and TLR2 were also upregulated [3].
The researchers in this study also found greater upregulation of both astrocytes, a type of glial cell involved in synaptic regulation, and microglia markers [3][35]. Glial fibrillary acidic protein (GFAP) is an intermediate filament used in the cytoskeleton of astrocytes [36]. More GFAP is typically found when astrocytes are activated in response to infection or injury. This protein was increased by 16-fold in mice with chronic ethanol doses [3]. CD11b is a cell surface protein that plays a role in cell movement and phagocytosis, a cellular process in which a cell takes in foreign particles and digests them [37] [38]. CD11b was upregulated 18-fold in chronic ethanol mice vs. control mice [3]. Boosted expression of these proteins involved in the neuroimmune response provides further evidence that chronic alcohol consumption may result in hyperactivation of the immune system.
Other studies have observed increased microglial proliferation after chronic alcohol consumption. In one study, adult male rats were given a 25% ethanol diet every 8 hours for 4 days [39]. During the next 56 days, the researchers periodically measured cell proliferation correlated with immunoreactivity using a tracer, finding it to be increased in several regions of the brain, including the hippocampus. After 56 days, the types of newly born cells in the brain were identified. Typically, about 75% of newly born neural cells become mature neurons, and approximately 10% become glial. However, in the brains of rats with an ethanol diet, only 24% of the new cells were mature neurons and 20% were astrocytes. The majority of the rest of the new cells were identified to be non-phagocytotic microglia. This shows that several binge doses within a small timespan can be responsible for markedly increased levels of microglia that persist for a few months [39].
This proliferation of microglia is also observed after cell death. During binge alcohol consumption, there is neuronal death, and neurogenesis is inhibited. Binge alcohol may cause cell death similar to an excessive brain injury, such as hippocampal damage [39]. Since cell death occurs in response to binge alcohol doses, proliferation of microglia in response to chronic alcohol is not unexpected [39].
Chronic alcohol abuse is known to increase susceptibility to infections [5]. In mice models, chronic ethanol consumption has been shown to reduce and impair the activity of B and T cells, which respond to infections [16]. One study found that the reduced number of B cells caused by chronic ethanol consumption lowered the ability for the immune system to fight the spread of malignant tumors [16]. Impairment of the immune system due to alcohol has also been found in humans. A study using blood from blood bank donors found ethanol increased T cell death due to a greater production of harmful molecules called reactive oxygen species (ROS) and inhibited metabolism of dysfunctional mitochondria [40]. It is important to continue the investigation of alcohol on not only minor infections but also the development of cancers.
From these results, it appears alcohol consumption causes different effects on the immune system depending on dosage and frequency of alcohol consumption [5]. Moderate alcohol consumption, approximately 1-2 drinks a day, has a stimulatory effect on the immune system causing increased phagocytotic activity, increased number of inflammatory cytokines, and increased number of immune cells. Even though several cytokines are increased after chronic ethanol exposure, the decrease in the number of T cells and B cells and the increased percentage of memory T cells and decreased percentage of naive T cells results in an increased susceptibility to infection [5].
Adolescent Response to Several Binge Doses
In a comparative study, researchers found that adolescent rats had a similar immune response to multiple binge doses of alcohol [41]. Partial microglial activation and proliferation of cells were observed after approximately 30 days in the hippocampus. Cytokine receptors are particularly dense in the hippocampus [32]. The hippocampus is also a region of profound change in an adolescent’s brain, making it particularly vulnerable to the effects of alcohol [42]. Several binge doses of alcohol have also been shown to impair neurogenesis in the hippocampus in rats [43].
Microglia are important in the development of neural circuitry and in myelination, so the disruption of microglia in adolescents could have severe consequences [44]. In response to activation, microglia release interleukins and ROS that can lead to neurodegeneration over time through TLR activation [45]. ROS are highly reactive and can damage complex structures such as fats, proteins, and DNA [16]. Interestingly, while many studies have noted the partial activity of microglia in response to binge drinking, others have observed a reduction in microglia in key regions of the brain right after the last binge ethanol dose, notably the hippocampus for both adults and adolescents [46]. This decrease in microglia likely occurs due to the harmful ROS that alcohol helps accumulate in the body. This suggests a harmful effect on adolescent neural development not only from hyperactive microglia but also from the loss of microglia. Although seemingly contradictory, these partially activated and malfunctioning microglia could themselves be targeted for degradation during heightened alcohol consumption, thus contributing to neurodegeneration [46].
Other studies have found further evidence that alcohol can impair cognition. One study used a rat model to test the relationship between adolescent alcohol consumption and key indicators of cognitive decline [47]. Increased expression of TLRs and various cytokines were associated with increased neuroinflammation and a greater loss of the protective myelin around neurons. A similar study observed the effects of alcohol consumption in both adult rats and postmortem human hippocampal tissue, finding increased hippocampal neurodegeneration in rats with binge-like alcohol consumption [48]. These studies indicate that binge-level alcohol consumption can have a negative effect on not only immunity but also cognitive development during adolescence and neurodegeneration later in life.
Alcohol Dependence
Alcohol use disorder (AUD) has long been attributed primarily to malfunctioning dopamine regulation, which is highly involved in the reward system [49]. Recent research has honed in on how the gut microbiome may also contribute to the risk of developing a dependence on alcohol. Several studies have indicated reduced biodiversity of the gut microbiome over time in response to chronic ethanol consumption [50]. This altered gut composition additionally leads to gut leakiness or the breakdown of the intestinal layer [51]. A study of the effects of alcohol dependence on inflammation noted that alcohol-dependent heightened levels of MyD88 and various inflammatory cytokines, as well as increased immune cells called peripheral blood mononuclear cells (PBMCs) [51]. In addition, upregulation of IL-1B and IL-8 has been associated with elevated alcohol consumption and cravings [52]. These findings indicate the inflammatory response to alcohol consumption may further contribute to addiction.
Adolescents have different responses to several binge doses of alcohol which may predispose them to alcohol addiction during adulthood. A study investigating the response of the reward system of adolescent rats to ethanol injection found that adolescent rats intermittently provided ethanol had increased subsequent consumption of alcohol, suggesting their preference for alcohol had grown [47]. The scientists proposed binge-level alcohol consumption during adolescence could lead to an impairment in cognition and therefore disrupt their development and make them more vulnerable to addiction in the first place [47].
Conclusion
Although drinking alcohol is often positively depicted in Hollywood and social media, it has several harmful effects on the neuroimmune system. In addition to the immediate disruption of cytokine levels that often results in sickness responses, chronic consumption may lead to continuous hyperactivation of the neuroimmune system. Not only does this hyperactivation lower general immunity, it also makes neuronal support cells such as microglia more vulnerable to degradation. This in turn reduces the body of support cells available for neurons, increasing the susceptibility to cognitive decline and even neurodegeneration. While chronic alcohol consumption can be harmful for everybody, it is particularly detrimental for adolescents, whose brains are less developed and more vulnerable to damage and addictive behaviors.
References
- U.S. Department of Health and Human Services. Alcohol’s effects on the body. National Institute on Alcohol Abuse and Alcoholism. https://www.niaaa.nih.gov/alcohols-effects-health/alcohols-effects-body
- Engelhardt, B., Vajkoczy, P. & Weller, R. The movers and shapers in immune privilege of the CNS. Nature Immunology, 18, 123–131 (2017). https://doi.org/10.1038/ni.3666
- Alfonso-Loeches, S., Pascual-Lucas, M., Blanco, A. M., Sanchez-Vera, I., & Guerri, C. (2010). Pivotal role of TLR4 receptors in alcohol-induced neuroinflammation and brain damage. The Journal of neuroscience: the official journal of the Society for Neuroscience, 30(24), 8285–8295. https://doi.org/10.1523/JNEUROSCI.0976-10.2010
- Dantzer R. (2018). Neuroimmune Interactions: From the Brain to the Immune System and Vice Versa. Physiological Reviews, 98(1), 477-504. https://doi.org/10.1152/physrev.00039.2016
- Barr, T., Helms, C., Grant, K., & Messaoudi, I. (2016). Opposing effects of alcohol on the immune system. Progress in neuro-psychopharmacology & biological psychiatry, 65, 242–251. https://doi.org/10.1016/j.pnpbp.2015.09.001
- Netea, G., Schlitzer, A., Placek, K., Joosten, B., & Schultze, L. (2019). Innate and Adaptive Immune Memory: an Evolutionary Continuum in the Host's Response to Pathogens. Cell host & microbe, 25(1), 13–26. https://doi.org/10.1016/j.chom.2018.12.006
- Espinoza, V. E., & Emmady, P. D. (2023). Histology, Monocytes. In StatPearls. StatPearls Publishing. https://pubmed.ncbi.nlm.nih.gov/32491550/
- Czerwińska-Błaszczyk, A., Pawlak, E., & Pawłowski, T. (2022). The Significance of Toll-Like Receptors in the Neuroimmunologic Background of Alcohol Dependence. Frontiers in psychiatry, 12, 797123. https://doi.org/10.3389/fpsyt.2021.797123
- Sampath, V. (2018). Bacterial endotoxin-lipopolysaccharide; structure, function and its role in immunity in vertebrates and invertebrates. Agriculture and Natural Resources. 52(2). https://doi.org/10.1016/j.anres.2018.08.002
- Blednov, A., Black, M., Chernis, J., Da Costa, A., Mayfield, J., & Harris, A. (2017). Ethanol Consumption in Mice Lacking CD14, TLR2, TLR4, or MyD88. Alcoholism, clinical and experimental research, 41(3), 516–530. https://doi.org/10.1111/acer.13316
- AbdAllah, B., Toraih, A., Al Ageeli, E., Elhagrasy, H., Gouda, S., Fawzy, S., & Helal, M. (2021). MYD88, NFKB1, and IL6 transcripts overexpression are associated with poor outcomes and short survival in neonatal sepsis. Scientific reports, 11(1), 13374. https://doi.org/10.1038/s41598-021-92912-7
- Justiz Vaillant, A., & Qurie, A. (2022). Interleukin. In StatPearls. StatPearls Publishing. https://www.ncbi.nlm.nih.gov/books/NBK499840/
- Crews, F. T., & Vetreno, R. P. (2016). Mechanisms of neuroimmune gene induction in alcoholism. Psychopharmacology, 233(9), 1543–1557. https://doi.org/10.1007/s00213-015-3906-1
- Kopitar-Jerala N. (2017). The Role of Interferons in Inflammation and Inflammasome Activation. Frontiers in immunology, 8, 873. https://doi.org/10.3389/fimmu.2017.00873
- Zanoni, I., & Granucci, F. (2013). Role of CD14 in host protection against infections and in metabolism regulation. Frontiers in cellular and infection microbiology, 3, 32. https://doi.org/10.3389/fcimb.2013.00032
- Wu, Z., Zhang, Z., Lei, Z., & Lei, P. (2019). CD14: Biology and role in the pathogenesis of disease. Cytokine & growth factor reviews, 48, 24–31. https://doi.org/10.1016/j.cytogfr.2019.06.003
- Warden, S., Azzam, M., DaCosta, A., Mason, S., Blednov, A., Messing, O., Mayfield, D., & Harris, A. (2019). Toll-like receptor 3 activation increases voluntary alcohol intake in C57BL/6J male mice. Brain, behavior, and immunity, 77, 55–65. https://doi.org/10.1016/j.bbi.2018.12.004
- Doremus-Fitzwater, L., & Deak, T. (2022). Adolescent neuroimmune function and its interaction with alcohol. International review of neurobiology, 161, 167–208. https://doi.org/10.1016/bs.irn.2021.08.006
- Ferrier, L., Bérard, F., Debrauwer, L., Chabo, C., Langella, P., Buéno, L., & Fioramonti, J. (2006). Impairment of the intestinal barrier by ethanol involves enteric microflora and mast cell activation in rodents. The American journal of pathology, 168(4), 1148–1154. https://doi.org/10.2353/ajpath.2006.050617
- Crews, T., Lawrimore, J., Walter, J., & Coleman, G., Jr (2017). The role of neuroimmune signaling in alcoholism. Neuropharmacology, 122, 56–73. https://doi.org/10.1016/j.neuropharm.2017.01.031
- Zou, Y., & Crews, T. (2014). Release of neuronal HMGB1 by ethanol through decreased HDAC activity activates brain neuroimmune signaling. PloS one, 9(2), e87915. https://doi.org/10.1371/journal.pone.0087915
- Gano, A., Doremus-Fitzwater, L., & Deak, T. (2016). Sustained alterations in neuroimmune gene expression after daily, but not intermittent, alcohol exposure. Brain research, 1646, 62–72. https://doi.org/10.1016/j.brainres.2016.05.027
- Qin, L., He, J., Hanes, N., Pluzarev, O., Hong, S., & Crews, T. (2008). Increased systemic and brain cytokine production and neuroinflammation by endotoxin following ethanol treatment. Journal of neuroinflammation, 5, 10. https://doi.org/10.1186/1742-2094-5-10
- Toda, C., Santoro, A., Kim, D., & Diano, S. (2017). POMC Neurons: From Birth to Death. Annual review of physiology, 79, 209–236. https://doi.org/10.1146/annurev-physiol-022516-034110
- Emanuele, V., LaPaglia, N., Kovacs, J., & Emanuele, A. (2005). The impact of burn injury and ethanol on the cytokine network of the mouse hypothalamus: reproductive implications. Cytokine, 30(3), 109–115. https://doi.org/10.1016/j.cyto.2004.11.004
- Doremus-Fitzwater, L., Gano, A., Paniccia, E., & Deak, T. (2015). Male adolescent rats display blunted cytokine responses in the CNS after acute ethanol or lipopolysaccharide exposure. Physiology & behavior, 148, 131–144. https://doi.org/10.1016/j.physbeh.2015.02.032
- Molteni, M., Gemma, S., & Rossetti, C. (2016). The Role of Toll-Like Receptor 4 in Infectious and Noninfectious Inflammation. Mediators of inflammation, 2016, 6978936. https://doi.org/10.1155/2016/6978936
- Dantzer R. (2009). Cytokine, sickness behavior, and depression. Immunology and Allergy Clinics of North America, 29(2), 247-64. https://doi.org/10.1016/j.iac.2009.02.002
- Doremus-Fitzwater, L., Buck, M., Bordner, K., Richey, L., Jones, E., & Deak, T. (2014). Intoxication- and withdrawal-dependent expression of central and peripheral cytokines following initial ethanol exposure. Alcoholism, clinical and experimental research, 38(8), 2186–2198. https://doi.org/10.1111/acer.12481
- Kim, J., Kim, W., Yoon, J., Choi, M., Kim, S., Go, J., Kim, K., & Jeong, J. (2003). Effects of alcohol hangover on cytokine production in healthy subjects. Alcohol, 31(3), 167–170. https://doi.org/10.1016/j.alcohol.2003.09.003
- van de Loo, J., Raasveld, J., Hogewoning, A., Zeeuw, R., Bosma, R., Bouwmeester, H., Lukkes, M., Knipping, K., Mackus, M., Kraneveld, D., Brookhuis, A., Garssen, J., Scholey, A., & Verster, C. (2021). Immune Responses after Heavy Alcohol Consumption: Cytokine Concentrations in Hangover-Sensitive and Hangover-Resistant Drinkers. Healthcare, 9(4), 395. https://doi.org/10.3390/healthcare9040395
- Verster C. (2008). The alcohol hangover--a puzzling phenomenon. Alcohol and alcoholism, 43(2), 124–126. https://doi.org/10.1093/alcalc/agm163
- Swift, R., & Davidson, D. (1998). Alcohol hangover: mechanisms and mediators. Alcohol health and research world, 22(1), 54–60. https://pubmed.ncbi.nlm.nih.gov/15706734/
- Spear, P. (2015). Adolescent alcohol exposure: Are there separable vulnerable periods within adolescence?. Physiology & behavior, 148, 122–130. https://doi.org/10.1016/j.physbeh.2015.01.027
- Koob, O. (2022). Astrocytes Imagined. Journal of integrative neuroscience, 21(4), 112. https://doi.org/10.31083/j.jin2104112
- Messing, A., & Brenner, M. (2020). GFAP at 50. ASN neuro, 12, 1759091420949680. https://doi.org/10.1177/1759091420949680
- Korf, M., Honarpisheh, P., Mohan, C., Banerjee, A., Blasco-Conesa, P., Honarpisheh, P., Guzman, U., Khan, R., Ganesh, P., Hazen, L., Lee, J., Kumar, A., McCullough, D., & Chauhan, A. (2022). CD11b high B Cells Increase after Stroke and Regulate Microglia. Journal of immunology, 209(2), 288–300. https://doi.org/10.4049/jimmunol.2100884
- Uribe-Querol, E., & Rosales, C. (2020). Phagocytosis: Our Current Understanding of a Universal Biological Process. Frontiers in immunology, 11, 1066. https://doi.org/10.3389/fimmu.2020.01066
- Nixon, K., Kim, H., Potts, N., He, J., & Crews, T. (2008). Distinct cell proliferation events during abstinence after alcohol dependence: microglia proliferation precedes neurogenesis. Neurobiology of disease, 31(2), 218–229. https://doi.org/10.1016/j.nbd.2008.04.009
- McTernan, M., Levitt, E., Welsh, A., Simon, L., Siggins, W., & Molina, E. (2022). Alcohol Impairs Immunometabolism and Promotes Naïve T Cell Differentiation to Pro-Inflammatory Th1 CD4+ T Cells. Frontiers in immunology, 13, 839390. https://doi.org/10.3389/fimmu.2022.839390
- McClain, A., Morris, A., Deeny, A., Marshall, A., Hayes, M., Kiser, M., & Nixon, K. (2011). Adolescent binge alcohol exposure induces long-lasting partial activation of microglia. Brain, behavior, and immunity, 25 Suppl 1(Suppl 1), S120–S128. https://doi.org/10.1016/j.bbi.2011.01.006
- Nagel, J., Schweinsburg, D., Phan, V., & Tapert, F. (2005). Reduced hippocampal volume among adolescents with alcohol use disorders without psychiatric comorbidity. Psychiatry research, 139(3), 181–190. https://doi.org/10.1016/j.pscychresns.2005.05.008
- Morris, A., Eaves W., Smith R., & Nixon K. (2010). Alcohol inhibition of neurogenesis: a mechanism of hippocampal neurodegeneration in an adolescent alcohol abuse model. Hippocampus, 20(5), 596-607. https://doi.org/10.1002/hipo.20665
- Melbourne, K., Chandler, M., Van Doorn, E., Bardo, T., Pauly, R., Peng, H., & Nixon, K. (2021). Primed for addiction: A critical review of the role of microglia in the neurodevelopmental consequences of adolescent alcohol drinking. Alcoholism, clinical and experimental research, 45(10), 1908–1926. https://doi.org/10.1111/acer.14694
- Yang, Y., Xue, X., Tian, H., Wang, X., Dong, X., Wang, F., Zhao, N., Yao, C., Cui, W., & Wu, F. (2014). Role of microglia in ethanol-induced neurodegenerative disease: Pathological and behavioral dysfunction at different developmental stages. Pharmacology & therapeutics, 144(3), 321–337. https://doi.org/10.1016/j.pharmthera.2014.07.002
- Marshall, A., McClain, A., Wooden, I., & Nixon, K. (2020). Microglia Dystrophy Following Binge-Like Alcohol Exposure in Adolescent and Adult Male Rats. Frontiers in neuroanatomy, 14, 52. https://doi.org/10.3389/fnana.2020.00052
- Pascual, M., Pla, A., Miñarro, J., & Guerri, C. (2014). Neuroimmune activation and myelin changes in adolescent rats exposed to high-dose alcohol and associated cognitive dysfunction: a review with reference to human adolescent drinking. Alcohol and alcoholism, 49(2), 187–192. https://doi.org/10.1093/alcalc/agt164
- Coleman, G., Jr, Zou, J., & Crews, T. (2017). Microglial-derived miRNA let-7 and HMGB1 contribute to ethanol-induced neurotoxicity via TLR7. Journal of neuroinflammation, 14(1), 22. https://doi.org/10.1186/s12974-017-0799-4
- Ma, H., & Zhu, G. (2014). The dopamine system and alcohol dependence. Shanghai archives of psychiatry, 26(2), 61–68. https://doi.org/10.3969/j.issn.1002-0829.2014.02.002
- Bull-Otterson, L., Feng, W., Kirpich, I., Wang, Y., Qin, X., Liu, Y., Gobejishvili, L., Joshi-Barve, S., Ayvaz, T., Petrosino, J., Kong, M., Barker, D., McClain, C., & Barve, S. (2013). Metagenomic analyses of alcohol induced pathogenic alterations in the intestinal microbiome and the effect of Lactobacillus rhamnosus GG treatment. PloS one, 8(1), e53028. https://doi.org/10.1371/journal.pone.0053028
- Keshavarzian, A., Choudhary, S., Holmes, W., Yong, S., Banan, A., Jakate, S., & Fields, Z. (2001). Preventing gut leakiness by oats supplementation ameliorates alcohol-induced liver damage in rats. The Journal of pharmacology and experimental therapeutics, 299(2), 442–448. https://pubmed.ncbi.nlm.nih.gov/11602653
- Leclercq, S., De Saeger, C., Delzenne, N., de Timary, P., & Stärkel, P. (2014). Role of inflammatory pathways, blood mononuclear cells, and gut-derived bacterial products in alcohol dependence. Biological psychiatry, 76(9), 725–733. https://doi.org/10.1016/j.biopsych.2014.02.003